EDF joins court challenge of FDA’s refusal to ban use of perchlorate in food contact materials
Tom Neltner, J.D., is Chemicals Policy Director and Maricel Maffini, Ph.D., Consultant
Today, EDF, represented by Earthjustice, joined with other public health advocates in filing a lawsuit to overturn the Food and Drug Administration’s (FDA) May 2017 decision, reaffirmed in April 2019 that allows the continued use of perchlorate[1], at concentrations up to 12,000 parts per million, in plastic packaging and processing equipment in contact with dry food. Perchlorate exposure is particularly dangerous for fetuses, infants, and young children, as it has been linked to developmental delays, reduced growth, and impaired learning capabilities. FDA relied on flawed reasoning while entirely ignoring important evidence developed by its own scientists revealing potentially serious risks resulting from ongoing use of perchlorate. We maintain that the intentional and unnecessary use of perchlorate in food contact materials should end.[2]
As with any litigation, we take this action reluctantly. We have long questioned FDA’s decisions that ignore evidence that endocrine disruptors like perchlorate can cause harm at levels the agency systematically dismisses as trivial. We have also pushed back on FDA’s decisions that allow toxic chemicals to be used in packaging and processing equipment that contact food ingredients multiple times from the farm to the grocery store shelf when the exposure estimate is based solely on the amount of the chemical that may migrate into food from the final product packaging. Agency assertions that its estimates are based on worst-case assumptions are misleading when they only consider a single contact. While FDA’s initial decision in November 2005 allowing the use of perchlorate-containing plastic raises all of these problems, the agency’s failure to address its own data and accompanying analysis by its own scientists that was published a decade later has left us with little choice but to act.
In December 2016, agency scientists published a peer-reviewed study showing that young children’s dietary exposure to perchlorate increased dramatically after FDA approved the use of the substance in plastic food contact materials in November 2005. The agency measured perchlorate in food samples collected as part of its regular Total Diet Study between 2008 and 2012 and compared it to those collected in 2005 and 2006. These dates are based on the federal fiscal year (FFY) that ends on September 30 (the importance of this detail will become clear later).
We carefully looked at the published data and found it strange that the increase in perchlorate exposure was greatest for infants and progressively leveled off as children reached ten years old. There was no change for teenagers and adults between these time periods. From the supplemental data, we could see that the increase appeared to primarily result from extraordinarily high perchlorate levels in samples of baby food dry cereal collected in FFY 2008-2012 that did not occur in similar samples collected in FFY 2005. We also noticed that, by coincidence, the only samples collected and analyzed for perchlorate in FFY 2005 were baby food. Almost all of the other food was collected after the agency allowed the perchlorate-containing plastic to be used (in November 2005). When we plotted the data, the difference was striking. See the figure below from our January 2019 blog with the results for baby food dry cereal collected before and after FDA decided to allow perchlorate to intentionally enter the food supply.
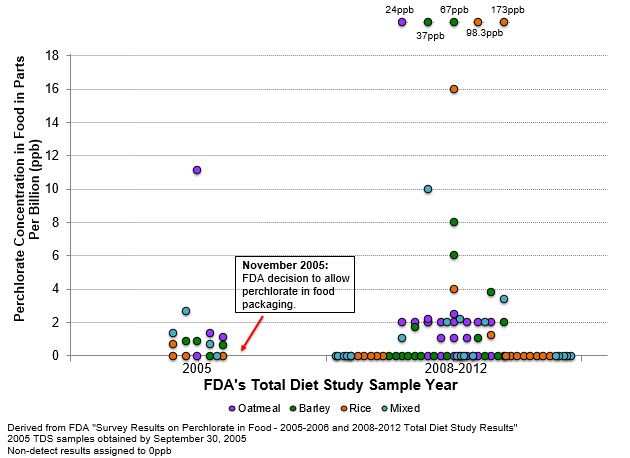
When we saw this stark difference, we immediately alerted the agency to the problem and assumed it would consider the data in the pending decision on the petition. However, the agency not only ignored the peer-review study, it released a webpage accompanying the decision stating that “Between 2008 and 2012, the FDA collected and tested a total of 5,464 food samples for perchlorate and found no overall change in perchlorate levels across foods compared to 937 samples collected between 2005 and 2006.” This statement is based on a misleading analysis of the data, because:
- Unlike the peer-reviewed study that estimated intake based on the types and amounts of foods people typically eat, the summary on the webpage treats more than 250 types of food equally, averaging consumption of foods across all the age groups (e.g., giving rice cereal marketed for babies and salami the same weight); and
- The webpage summary lumps baby food collected in FFY 2005, before the agency’s November 2005 decision, with foods collected in FFY 2006 (none of which was baby food). This further clouds the interpretation of the data.
By taking this flawed approach, FDA concluded that there were no changes in levels of perchlorate in food, but didn’t explain why young children’s exposure had increased and didn’t consider the implications.
We included our analysis and additional scientific arguments when we filed our formal objections to FDA’s May 2017 denial of the advocates’ petition assuming that the agency would reconsider its decision and address the implications of its own data. Instead, the FDA took almost two years and ultimately rejected our objections on procedural grounds. Even in a letter FDA sent us the day after the decision was announced that attempted to explain the rationale of its decision, the agency continued to ignore the data.
We will explain the shortcomings of FDA’s analysis later, but in essence, it reveals the more systemic flaws in the agency’s process to estimate exposure to toxic chemicals in food contact materials, especially ones where even small amounts can disrupt the normal development of immature organs and systems such as the brain, reproductive, immune and endocrine systems.
Conclusion
It has been five years since the initial petition was submitted. Since 2017, we have alerted FDA of its own data and the agency has consistently failed to address it. Now we are suing to force FDA to move to the next step, which, hopefully will be a decision that adequately addresses the flaws in a decision FDA made 14 years ago when it allowed perchlorate to be purposely introduced into the food supply. In the meantime, we will continue to call on food manufacturers, especially baby food companies, to consider this risk and take action on their own to avoid this unnecessary exposure to perchlorate.
[1] In 2005, FDA authorized CIBA Specialty Chemicals, now BASF, to sell perchlorate as anti-static agent. https://www.accessdata.fda.gov/scripts/fdcc/index.cfm?set=TOR&id=2005-006
[2] In 2014, public health advocates petitioned FDA to revoke its authorization for the use of perchlorate in materials that contact food. https://www.regulations.gov/docket?rpp=100&so=DESC&sb=docId&po=0&D=FDA-2015-F-0537