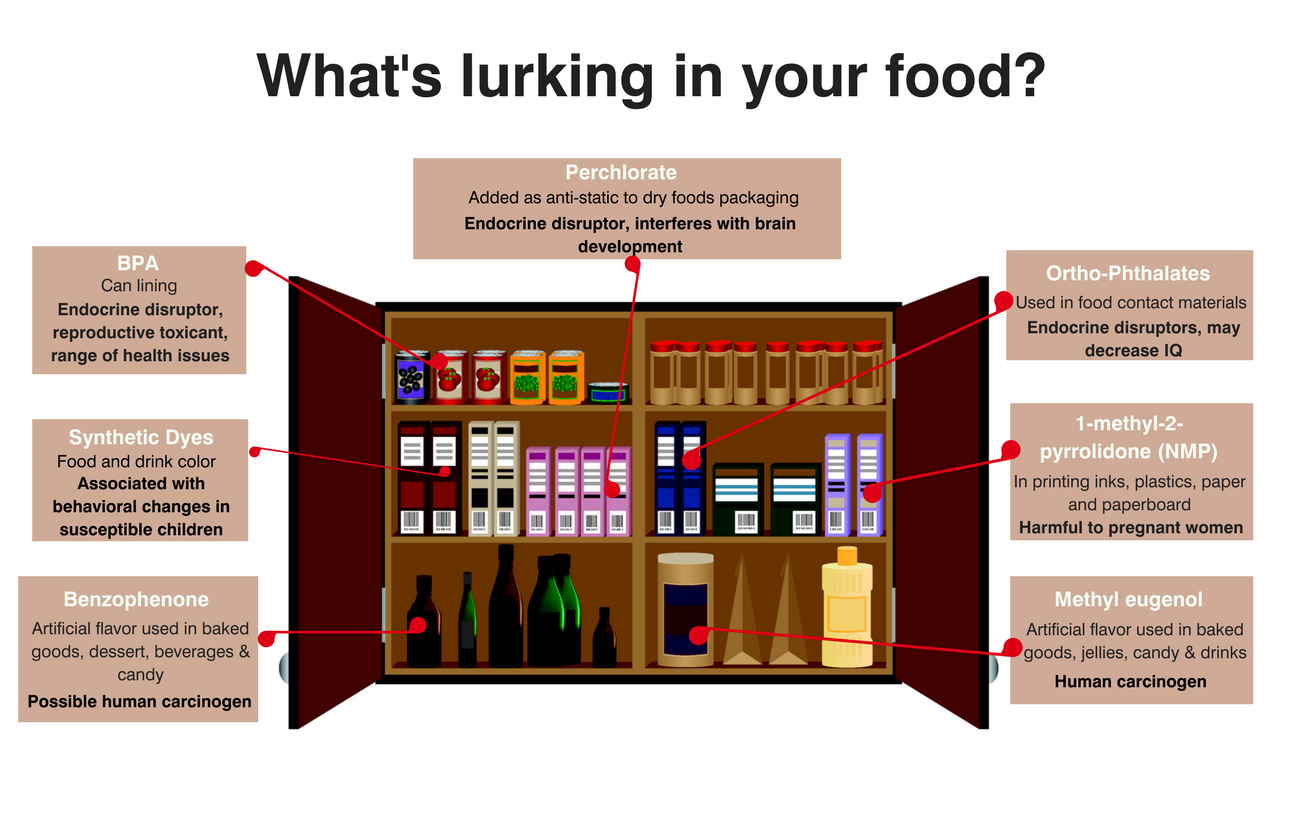
What Happened?
FDA estimates that, each year, food companies designate 82 new food chemicals as “GRAS” (Generally Recognized as Safe) for use in food. On average, FDA reviews only 64 of those new chemicals for safety. For the remaining 18 chemicals in FDA’s estimate, the companies making and marketing them for use in food or in the food-production process choose not to seek a voluntary review by FDA.
In comments to the agency, we said we think FDA’s estimate may be too low – and the number of new chemicals added to food that bypass FDA review may be as high as 130 new food chemicals a year (significantly higher than 18). This is based on searches of company marketing claims. In an 8-week period, we identified 10 chemicals claimed as GRAS without a submitted notice to FDA seeking voluntary review. (Please see our comments for a full explanation of our estimate.)
Why It Matters
I want to throw in chemical safety as another really, really important area for the future – for humankind, really – and where science is evolving rapidly.—Robert Califf, MD, FDA Commissioner
FDA’s review of GRAS safety determinations is critical to ensure food chemicals are safe. When agency scientists receive GRAS notices, they closely review them and ask notifiers tough questions about the safety of the chemical alone—and the potential consequences when that chemical is combined with other chemicals on the market.
But FDA allows companies to withdraw their notices – which they do in about 17% of cases. Sometimes companies fix the problems and resubmit the notice. FDA does not require companies to do this, however. Too many simply continue to market the chemical for food uses as GRAS without seeking further FDA review. That is in addition to the GRAS safety determinations companies choose never to file, which leads to chemicals entering our food system without any FDA notice or review at all.
Our Take
While the GRAS system is clearly broken (something we’ve blogged about at length and the Washington Post covered in-depth back in 2014), FDA does retain the ability and the duty to monitor the marketplace to ensure unsafe chemicals that food companies claim as GRAS are kept off the market. Unfortunately, the agency appears to lack any system to ensure that monitoring takes place. The only examples of agency action to pull industry-certified GRAS products off the market have been caffeinated alcoholic beverages and partially hydrogenated oils (aka artificial trans fat). Both were prompted, in part, by state and media calls for action.
Examples of inaction, however, are numerous and include: tara flour, COZ corn oil, Apocynun ventron, gamma aminobutyric acid (GABA), aquaequorin/Prevagen, and many more.
One of the key breakdowns that contributes to FDA’s failure to monitor is the lack of coordination between the regulatory programs in the Center for Food Safety and Applied Nutrition (CFSAN) and the inspection/enforcement programs in the Office of Regulatory Affairs (ORA).
Next Steps
In January 2023, FDA Commissioner Califf announced a proposal to reorganize the food safety program in response to stakeholder calls for action after the infant formula debacle and to a recent review of the agency by the Reagan-Udall Foundation. This review noted that one key step is to appoint a new Deputy Commissioner for Human Food. This person would have greater responsibility to coordinate efforts between CFSAN and ORA. The Commissioner’s proposal has been strongly criticized since there would be no clear line of authority between the new Deputy Commissioner and ORA.
But Commissioner Califf has stated that chemical safety is a priority, telling a reporter that “I want to throw in chemical safety as another really, really important area for the future – for humankind, really – and where science is evolving rapidly.”[1]
Fixing GRAS is an important step to rebuild consumer confidence and reduce the ongoing risk to public health. Until the broken GRAS system is fixed, FDA will continue to be hamstrung in preventing health risks posed by chemicals of unknown safety. Until the system is fully fixed—which includes ensuring that no chemicals enter our food system without notice to, and review by, the agency—FDA needs to be coordinating with ORA and CFSAN to proactively monitor and enforce GRAS evaluations on chemicals entering the market to ensure they are actually safe.
Go deeper: You can learn more from these resources:
NOTES
[1] FoodFix, January 31, 2023, edition.