By Maricel Maffini, PhD, Consultant, and Tom Neltner, JD
What Happened
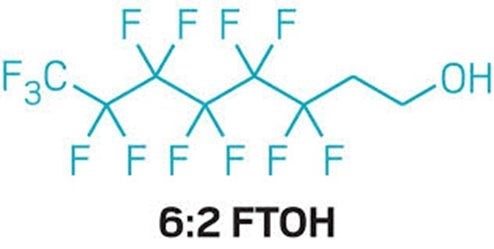
In December 2023, FDA’s scientists published a new study showing that when pregnant rats ingest a form of per- and polyfluorinated alkyl substance (PFAS) called 6:2 fluorotelomer alcohol (6:2 FTOH) their bodies break it down into other PFAS that reach the fetuses and biopersist in the mother and the pups.
The study also showed that the body of a non-pregnant animal produces different breakdown products that also biopersist. This study is the latest evidence that the assumptions made about the safety of short-chain PFAS (chemicals with fewer than 8 carbons) have been wrong.
Why It Matters
In July 2020, FDA announced that four companies agreed to a voluntary phase-out of 6:2 FTOH following the agency’s demonstration of safety concerns. [PDF, 2.4MB]
Between 2006-2016, FDA had authorized the use of 6:2 FTOH in paper and paperboard packaging—often as substitutes for long-chain PFAS such as PFOA—without information on the potential biopersistence of the chemicals. In the phase-out agreement, FDA allowed food packaging containing the toxic chemicals to continue to be sold without limit until December 2023—more than three years after its scientists had showed the chemicals biopersist and pose a risk to human health.
In a previous blog, we discussed how, for more than a decade, industry led people to believe that replacing long-chain PFAS with shorter chains would be ‘more favorable’ to human health and the environment without fully disclosing information.
For example, to ensure chemicals are safe to be consumed in the diet, it is critical to understand how the body responds to them. Most toxicity tests are designed with the assumption that chemicals are eliminated quickly (e.g., within 24-48 hours) and therefore the short exposure is unlikely to cause harm.
Although earlier publications showed short-chain PFAS remaining in the body for months, FDA publicly acknowledged this issue only in 2018. Two additional publications by the same FDA scientists confirmed that breakdown products from 6:2 FTOH biopersist in the body and were more toxic than industry-funded studies indicated.
Our Take
Unfortunately, not much has changed in FDA’s approach. The agency continued to authorize new PFAS for food contact uses until as recently as 2019, concluding that those uses were reasonably certain to cause no harm, consistent with FDA’s rule.
Although neither the safety information that FDA relied on nor FDA’s review are available, we can assume that FDA did not request information on either biopersistence or toxicity, other than genotoxicity. We base this assumption on our review of many PFAS authorizations EDF obtained through a Freedom of Information Act (FOIA) request.
Those authorizations indicate FDA is continuing to rely on assumptions that may again be proven wrong and harmful to public health. In 2021, recognizing the agency’s shortcoming, 11 organizations, including EDF, petitioned FDA to ban all PFAS unless there is affirmative evidence that these substances do not biopersist in the human body. Well over two years later, however, FDA has yet to respond to this citizens’ petition.
FDA’s latest evidence, consistent with other published data, shows that chemicals thought to be quickly eliminated actually remain in the body and transfer from mother to offspring during pregnancy. A human study has also showed that maternal exposure to short-chain PFAS affects cardiovascular development in children. In addition, a recent article highlighting the abundance of short- and ultra-short PFAS (substances with two or three carbons) in humans makes it imperative for FDA to respond to the petition.
FDA’s actions authorizing PFAS without relevant information about potential biopersistence have significantly contributed to Americans’ exposure to these toxic chemicals and undoubtedly increased their health risk. It took the agency more than a decade to conclude that these substances are not safe while it continued to allow PFAS use for plastics and other purposes.
Strangely, the agency does not monitor the food supply for the substances it continues to allow in contact with food. Sadly, the agency has chosen to keep the blinders on about its regulatory obligations to protect the public from unsafe substances.
Next Steps
We will continue to engage with FDA and press the agency to recognize that the safety of all uses of PFAS cannot be ensured without solid evidence that PFAS do not accumulate in the body.
Go Deeper
Read our blogs on PFAS in food.
Download a PDF of this blog post [340KB]