Maricel Maffini, Ph.D., Consultant and Tom Neltner, J.D., Chemicals Policy Director
In January 2018, US Food and Drug Administration (FDA) scientists published a peer-reviewed journal article stating a commonly used raw material to make greaseproof paper is likely to persist in the human body. FDA scientists’ sophisticated analysis and remarkable conclusion raises questions about the broad assumption that short-chain perfluorinated alkyl substances (PFAS), as a class, did not accumulate.
Strangely, two recent reviews funded by the FluoroCouncil, ignored FDA scientists’ study even though it was published ten months before the industry group submitted their analysis for peer-review. The peer reviewers appear to have missed the omission as well. As a result, the industry evaluations continue to perpetuate the flawed assumptions, concluding that perfluorohexanoic acid (PFHxA) and related short-chain PFAS “present negligible human health risk” and that this substance alone is a suitable marker for the “safety of fluorotelomer replacement chemistry.”
In this blog, we discuss the differences between the studies and the implications of the discordance between FDA’s and industry’s conclusions for the safety assessment of short-chain PFAS.
What do we know about short-chain PFAS?
With the phase-out of long-chain PFAS to make water- and grease-proof materials, companies shifted to short-chain fluorotelomer-based chemistry.[1] These new raw materials are used to build polymers and include chemicals that contain six fully-fluorinated carbon groups with additional non-fluorinated carbons. These molecules are usually known as C6 and a common starting material for polymers is a 6:2 fluorotelomer alcohol (6:2 FTOH) that has six fully-fluorinated carbons and two non-fluorinated carbons with an alcohol on the non-fluorinated end.
Industry expected that these C6 compounds, among them 6:2 FTOH and its main manufacturing impurity perfluorohexanoic acid (PFHxA), would: 1) be less toxic than long-chain PFAS such as 8:2 FTOH, PFOA and PFOS; and 2) not accumulate in the body.
However, these expectations do not hold up under scrutiny. A 2015 study by an FDA scientist concluded that “significant data gaps remain” about the toxicity of the 6:2 FTOH, and in the 2018 study, the agency scientists raised the potential for biopersistence.
Significance of FDA’s conclusion about potential biopersistence of C6 fluorotelomer alcohol
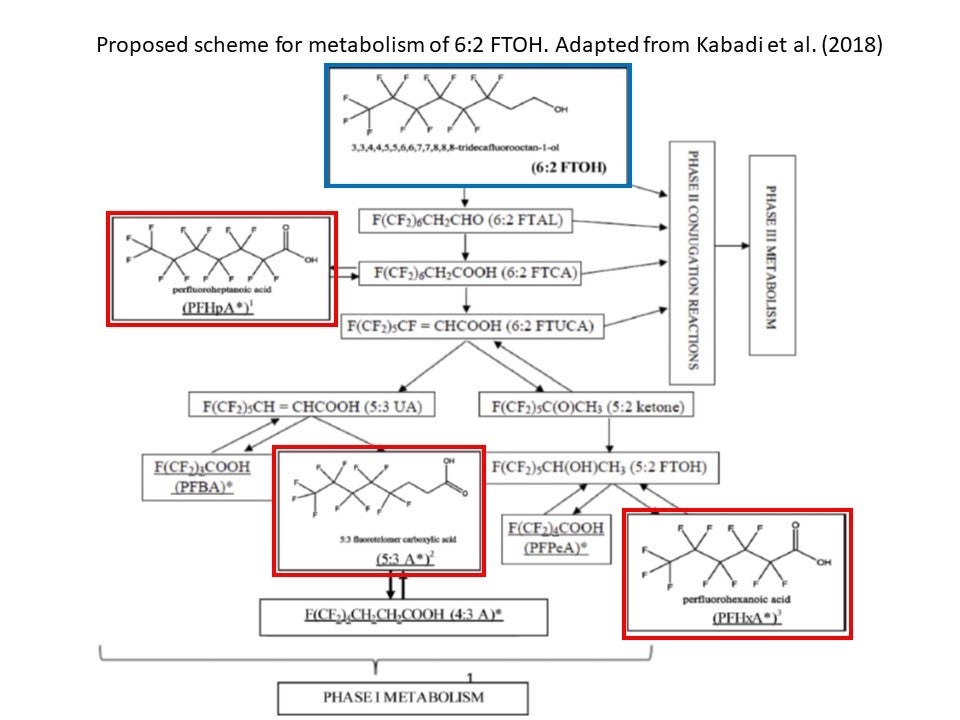
FDA scientists’ thorough evaluation of publicly available[2] animal and human exposure data on 6:2 FTOH provided important insight into the body’s transformation of the chemical. They identified three metabolites, namely the PFHxA mentioned above, 5:3 fluorotelomer carboxylic acid (5:3 A) and perfluoroheptanoic acid (PFHpA) that could be used as markers of 6:2 FTOH exposure. For each metabolite, they also provided internal exposure estimates. The figure below shows 6:2 FTOH (in blue box) and how the body converts it to the three metabolites (in red boxes).
As a result of their analysis, FDA scientists identified 5:3 A as an important biomarker for the potential biopersistence of 6:2 FTOH because:
- 5:3 A had the highest internal exposure and the slowest elimination by the body; and
- 5:3 A’s elimination was reduced when exposure to 6:2 FTOH increased.
FDA’s scientists also concluded there are sex-based differences in the elimination of the other two metabolites, PFHxA and PFHpA, in animal studies. Although human data was only available in men, the difference observed in animals could mean that men and women may have different internal exposures and, therefore, experience different toxicity.
FluoroCouncil-funded reviews reached a different conclusion
In January 2019, two reviews (HERE and HERE) funded by the FluoroCouncil were published concluding that:
- PFHxA “is less hazardous to human health than PFOA”;
- “PFHxA and related fluorotelomer precursors currently appear to present negligible human health risk to the general population”; and
- PFHxA is not expected to bioaccumulate due to its “rapid and nearly complete elimination” from the body.
These reviews evaluated the toxicology, exposure and biomonitoring data available for PFHxA. The analysis included the estimation of a toxicity reference dose and drinking water and residential groundwater screening levels. The overall conclusion was that “PFHxA levels currently present in the environment are well below levels that may present a concern for human health.”
The main difference between these reviews and the study by FDA scientists is that the industry-funded scientists focused exclusively on PFHxA. Their goal was to review the literature relevant to risk assessment to answer questions regarding “potential human health risks associated with exposure to fluorotelomer-based products” using PFHxA as a reference chemical for the entire short-chain PFAS class.
The two industry-funded reviews reported that PFHxA has been measured in water, soil, household dust, human serum, plasma, whole blood, urine, breast milk, and food with various frequencies. And that the substance is environmentally persistent, mobile and may accumulate in the leaves and fruits of plants. The reviews also reference a 2013 publication estimating that the mean half-life in humans is 32 days. In other words, it may take a month on average for half the amount present in the body to be eliminated.
Although the industry-funded reviews narrowly focused on a single chemical, the authors extended their conclusion to the entire fluorotelomer-based chemical process when they say that PFHxA is a “suitable marker for the safety of fluorotelomer replacement chemistry used today.” That is quite a bold statement that was not fully explained.
Following their reasoning, any other short-chain PFAS used in fluorotelomer-based products would be assumed to be as safe as PFHxA, including 6:2 FTOH. That assumption, however, appears to be flawed based on FDA scientists’ study showing that 6:2 FTOH metabolite 5:3 A is an important biomarker for the potential biopersistence of 6:2 fluorotelomer alcohol.
Conclusion
The study by FDA scientists has the potential to be a game changer in the safety assessment of short-chain PFAS. Based on their conclusions, safety studies must:
- Assess how the body breaks down these chemicals and how fast they are eliminated; and
- Be redesigned to account for biopersistence by including long-term exposures and exposures during development.
A decade ago, industry led us to believe that the new technology replacing toxic long-chain PFAS would be “more favorable” to human health and the environment. As a result, FDA has been approving short-chain fluorotelomer chemicals to make polymers for use in contact with food without information on the potential biopersistence of the chemicals themselves or their metabolites.
As we have noted in previous blogs, it is time to start making decisions on chemicals’ safety based on scientific evidence – not on assumptions. For PFAS, FDA needs to reassess the safety and environmental impacts of these chemicals for use as food contact substances. Until that review is complete, companies should avoid using products treated with the chemicals.
[1] For more information see Buck et al. 2011. Perfluoroalkyl and Polyfluoroalkyl Substances in the Environment: Terminology, Classification, and Origins. Integrated Environmental Assessment and Management — Volume 7, Number 4—pp. 513–541.
[2] Except for Nilsson et al. (2013), all the publications were by DuPont scientists. Russell et al. (2015). Himmelstein et al. (2012). DeLorme et al. (2011). Himmelstein et al. (2012).