Tom Neltner, Chemicals Policy Director, Maricel Maffini, consultant, and Tom Bruton with Green Science Policy Institute.
Update August 11, 21 – Added FDA’s Response to FOIA.
Results from an Environmental Protection Agency (EPA) investigation into PFAS-contaminated pesticides have much broader, concerning implications for food, cosmetics, shampoos, household cleaning products, and other consumer products, as well as recycling. This investigation, first announced earlier this year, found that fluorinated high-density polyethylene (HDPE) containers used for pesticide storage contained a mix of short and long-chain per- and polyfluorinated alkyl substances (PFAS), including PFOA, that leached into the product. From what EPA can tell, the PFAS were not intentionally added to the HDPE containers but are hypothesized to have been produced when fluorine gas was applied to the plastic.
Since EPA released its investigation, we have learned the disturbing fact that the fluorination of plastic is commonly used to treat hundreds of millions of polyethylene and polypropylene containers each year ranging from packaged food and consumer products that individuals buy to larger containers used by retailers such as restaurants to even larger drums used by manufacturers to store and transport fluids.
The process of polyethylene fluorination was approved by the Food and Drug Administration (FDA) in 1983 for food packaging to reduce oxygen and moisture migration through the plastic that would cause foods to spoil. The fluorination process forms a barrier on the plastic’s surface and it also strengthens the packaging.
Fluorination of plastic leading to the inadvertent creation of PFAS may be another reason these ‘forever chemicals’ show up in many unexpected places. This significant source of PFAS contamination needs to be addressed. Much remains to be resolved as FDA and EPA actively investigate this new source of PFAS; however, preventive steps need to be taken quickly, especially since other PFAS-free barrier materials are available as alternatives.
Growing evidence links PFAS to a wide range of serious health effects – from developmental problems to cancer.
FDA’s approval of fluorine gas treatment of polyethylene
FDA rules, promulgated in 1983, allow the use of fluorine gas to treat polyethylene food-contact articles in amounts that produce up to 5,000 parts per billion (ppb) of total fluorine in the food in the container.[1] The rule states that the process affects only the surface of the polyethylene and leaves the interior of the plastic unchanged.
In practice, the fluorine gas substitutes the hydrogen molecules on the plastic’s surface with fluorine, effectively creating a barrier to moisture and oxygen migration through the polyethylene. The newly created barrier also makes the plastic stronger by preventing the contents from penetrating the plastic and making it softer. We submitted a Freedom of Information Act (FOIA) request to FDA in May to obtain the documents surrounding FDA’s 1983 approval and the basis of its determination that the use was safe. We will share more information when we get the agency’s response.
The 5,000 ppb level of total fluorine in a container’s food translates into extremely high levels of PFAS exposure for consumers. Using PFOA as an example, this limit would allow up to 7,260 ppb[2] of PFOA in the food. To provide context, consider a one-liter bottle of fluorinated HDPE where only 1% of the PFAS made from the fluorination process was PFOA; an adult consuming the one-liter of beverage each day would be exposed to more than 300 times the Minimal Risk Level[3] that FDA, EPA, and Centers for Disease Control and Prevention have established for intermediate-duration exposures. And this wouldn’t include the risks from other types of PFAS also generated during fluorination or exposures to the substances from other sources.
We have been told by packaging experts and found in marketing materials[4] that fluorination is also used on polypropylene, but we cannot find any FDA approval for the use. If this is happening without an FDA authorization, food manufacturers could be self-certifying the use of fluorine gas as Generally Recognized as Safe (GRAS) without FDA review, a practice that FDA allows and that EDF and Center for Food Safety have challenged in court.
EPA identified eight PFAS from HDPE containers for mosquito control pesticide
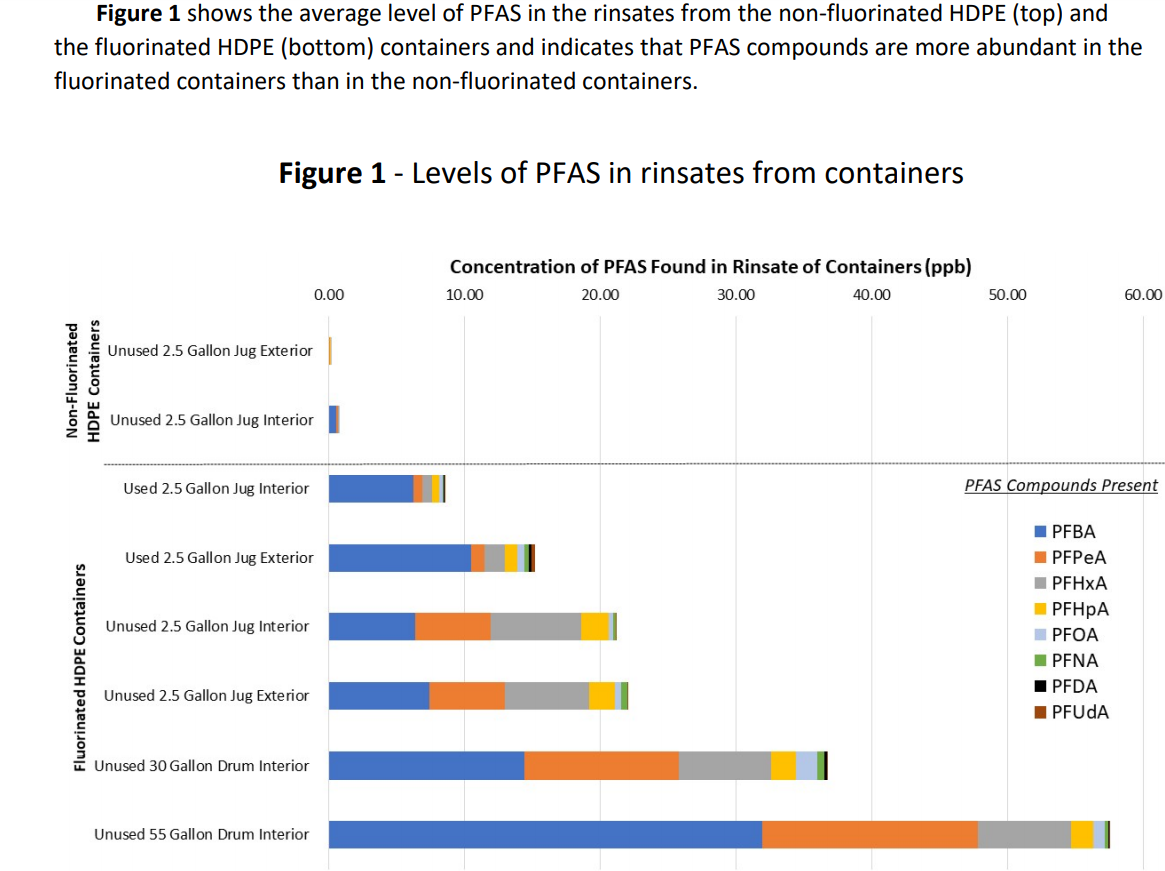
In its investigation into the PFAS-contaminated pesticides, EPA suspected the fluorinated HDPE containers as a potential source of the contamination and tested various sizes of used and unused fluorinated and non-fluorinated containers that was representative of the supply chain for pesticides (see photos below). In March 2021, EPA released final results of its investigation, and confirmed that the fluorination process is what produced the PFAS.
EPA rinsed eight HDPE containers for a very short time – only a minute – at room temperature with a small amount of methanol. Then it tested the rinsate for PFAS and positively identified eight PFAS with carbon-chain lengths between three and ten fully fluorinated carbons, including PFOA. The total level of the PFAS found ranged from 20-50 ppb in four unused containers; two used containers had lower, but still significant levels.
In contrast, the non-fluorinated containers had 1 ppb or less. See Figure 1 below from the EPA report for more detail. During this extremely short contact at room temperature, between 1 and 17 micrograms (µg) were extracted in the rinsate. If the contact time were longer and temperature was hotter to more closely represented actual conditions of storage and transport, the amount extracted would almost certainly have been greater.
In its conclusion, the agency stated that it “believes that through the fluorination process of HDPE containers, PFAS compounds may be formed and then partly leach into the products inside the containers.” EPA further explained that:
- During the fluorination process, HDPE containers are subjected to fluorine elemental gas at pre-determined concentrations and under elevated temperatures; and
- The length of time and the conditions under which the product is stored in the fluorinated containers could affect the leaching potential, and consequently the concentration of PFAS found in the products.
EPA also indicated that in future studies it will test these materials under a variety of different conditions including other solvents, different contact times and temperatures and different product storage time to better understand what impact fluorination has on plastic containers and their contents.
A 2011 study shows fluorine gas treatment creates PFAS, including PFOA
As we researched the issues raised by the EPA report, we learned of an excellent study published over a decade ago by Amy Rand and Scott Mabury at University of Toronto that raised the alarm about how fluorination can inadvertently create PFAS, at levels much greater than EPA found in their investigation. The difference is most likely due to the longer contact times – two hours compared to one minute – and higher temperatures that better simulate typical shipping conditions.
The researchers bought 20 one-liter fluorinated HDPE bottles from two separate firms: Fluoro-Seal International (now Inhance Technologies), which produced bottles with five degrees of fluorination; and Air Products and Chemicals/Airopak which made bottles with only one fluorination level.
After extracting the plastic bottles with methanol at elevated temperatures for two hours, they tested for nine PFAS with fully fluorinated carbon chain lengths between one and nine. The bottles with higher degrees of fluorination (called F5) had the highest amounts of PFAS and the PFAS were longer in length (see the table below for more detail). Longer PFAS tend to bioaccumulate in the human body longer.
The table below compares the study findings.
| Study | Method | Type of fluorinated HDPE container | # PFAS measured | PFAS carbon-chain lengths detected | Estimated PFAS concentration in contents |
|---|---|---|---|---|---|
| EPA | Methanol extraction from plastic; about one- minute rinse | 2.5 to 55 gallons | 8 | 4-11 | 0.05 to 1.2 ppb |
| Rand and Mabury 2011 | Methanol extraction from plastic; 2 hours at 65◦C | 1 L with lowest fluorination | 9 | 2-4; 9* | 5.1 ppb** |
| 1 L with highest fluorination | 9 | 2-10 | 70 ppb*** | ||
| *C5 and 7 were below the limit of quantification; C6,8 and 10 were not detected. **113 ng * 0.5 * 1200 cm2 inside and outside per kg of contents * 0.001 µg/ng = 70 ppb. ***8.5 ng * 0.5 * 1200 cm2 inside and outside per kg of contents * 0.001 µg/ng = 5.1 ppb. |
|||||
They also stored water in fluorinated bottles for one year and then tested samples of the water for the same PFAS. The average total PFAS was 188 ppb and the PFAS were predominantly short-chain[5]; no PFOA or longer-chain PFAS were detected possibly due to their low affinity for water compared to methanol.
Implications for companies
EPA indicated it has reached out to industry and trade organizations “to raise awareness of this emerging issue and discuss expectations of product stewardship.” The presence of PFAS in common plastics has also raised questions about recyclability claims as these types of plastic are commonly recycled.
As companies start to phase out PFAS from their products, they should keep in mind that assurances by their suppliers that PFAS are not intentionally used may not be sufficient. Fluorination of plastic surfaces generates PFAS that are likely to leach into the packaging content, but these PFAS are not intentionally used. Companies should ask suppliers whether they fluorinate the plastic containers.
FDA and EPA must address unanswered questions and take action
Given the reported range of products using plastic containers treated with fluorine, we are pleased to see that EPA and FDA are actively coordinating in evaluating the situation and taking the next steps.
We believe that FDA needs to conduct two types of safety review. The first is whether the product’s use is so widespread that it constitutes an imminent hazard to public health that warrants a recall. To do this assessment, FDA should work with EPA to quickly identify and investigate container manufacturing facilities that store significant quantities of fluorine gas and have had to submit risk management plans under the Clean Air Act.
The second review needed is evaluating whether there is sufficient evidence that the fluorine gas treatment approved by the agency in 1983 for polyethylene is safe or if the agency should stop its continued use as a food contact substance. For this purpose, 21 C.F.R. 170.3(i) defines safe to mean “there is a reasonable certainty in the minds of competent scientists that the substance is not harmful under the conditions of its intended use” after considering three factors that include “the cumulative effect of the substance in the diet, taking into account any chemically or pharmacologically related substance or substances in such diet.”
Given the evidence we see from EPA 2021 study and Rand and Mabury’s 2011 study, we cannot see how FDA could maintain the fluorination treatment process is safe, and we hope that the agency will take swift action to ban the practice to protect public health.
______
[1] The regulation uses parts per million. We converted it to parts per billion in order to use a consistent set of units.
[2] 7264 µg/kg of food (ppb) = (5000 µg F / kg of food) * (1 µmol F / 19 µg F) * (1 µmol PFOA / 15 µmol F) * (414.07 µg PFOA / µmol PFOA).
[3] Oral Intermediate Minimal Risk Level (MRL) is 3×10-6 milligrams of PFOA per kilogram of body weight per day established by the CDC’s Agency for Toxic Substances and Disease Registry in May 2021 Toxicological Profile for Perfluoroalkyls. See Table 1-2 and 1-3. FDA adopted the MRLs in June 2021. An Intermediate MRL is based on 15 to 364 days of exposure.
[4] Polypropylene is marketed as a candidate for direct fluorination https://www.berlinpackaging.com/fluorination/
[5] Trifluoroacetic acid (C2) and perfluoropropanoic acid (C3) comprised 80% of the total PFAS. We calculated 188 ppb by multiplying 314 ng/cm2 times 600 cm2 for inside surface of 1L bottle times 1000 ng/microgram.