Tom Neltner, J.D., Chemicals Policy Director and Maricel Maffini, Ph.D., Consultant
The Food and Drug Administration (FDA) released a study in April estimating young children’s exposure to lead and cadmium from their diets and identifying food groups that are a significant source of these heavy metals. The study used data from the agency’s Total Diet Study (TDS) program for 2014 to 2016 and the Center for Disease Control and Prevention’s (CDC) What We Eat in America (WWEIA) Survey for 2009 to 2014.[1]
The study is a reminder of how pervasive heavy metals are in children’s diets and that, while the levels are relatively low, the cumulative exposure is significant. Based on FDA’s analysis (Table 1 below), we estimate that about 2.2 million children exceeded the agency’s maximum daily intake (MDI) for lead at a given time. The results for cadmium are new and worrisome, with estimated daily intake (EDI) levels that are 3 to 4 times greater than lead. And while FDA has not yet set an MDI limit for cadmium, the average young child exceeds most of the relevant daily exposure limits set by other agencies. Clearly, cadmium warrants greater attention, but note that the evidence of neurotoxicity is still emerging.
Table 1: Young children’s estimated dietary intake (EDI) of lead and cadmium based on FDA’s TDS results for years 2014 to 2016 (based on hybrid method)
| Age Group | Lead Mean EDI | Lead 90th Percentile EDI | Cadmium Mean EDI | Cadmium 90th Percentile EDI |
|---|---|---|---|---|
| 1-6 years | 1.8 µg/day | 2.9 µg/day | 6.8 µg/day | 11.0 µg/day |
| 1-3 years | 1.7 µg/day | 2.6 µg/day | 5.8 µg/day | 9.7 µg/day |
| 4-6 years | 2.0 µg/day | 3.1 µg/day | 7.8 µg/day | 12.1 µg/day |
| Limits | FDA’s MDI is 3.0 | No MDL set by FDA. Intake exceeded most limits set by other agencies | ||
Steady decline in lead exposure but more work is needed
In 2018, the agency reaffirmed that there is no safe level of lead in blood and designated 3.0 μg/day as the MDI for lead. The agency referred to the MDI as the “Lead Interim Reference Level” and derived it from a value set by the Centers for Disease Control and Prevention (CDC) in 2012. Using FDA’s “hybrid method”[2] as the best estimate of exposure, 10% of the approximately 24 million one- to six-year-old children in the population were exposed to more than 2.9 μg/day of lead in their diet. We rounded down and estimated that 2.2 million young children exceeded the MDI for lead. This number is greater than the 1.2 million children we previously estimated using a different method and more conservative assumptions.
FDA reported that fruits, grains, dairy, and foods made from multiple ingredients were sources of almost 85% of the dietary intake of lead for young children. The agency found detectable levels of lead in all TDS samples of 18 foods collected from 2014 to 2016:
- Five canned foods: sweet potatoes, fruit cocktail, apricots, peaches, and pears;
- Four types of baby food: teething biscuits, sweet potatoes, peach cobbler/dessert, and macaroni and cheese;
- Five chocolate-containing foods: cake, brownies, cookies, candy, and syrup; and
- Four other foods – oat-ring cereal, raisins, wine, and liver.
Cadmium contamination is more frequent and exposures are greater than lead
Cadmium was more commonly found at detectable levels than lead. The agency found detectable levels of cadmium in all samples in 140 food types, including 21 baby foods, compared to 18 for lead. Despite this pervasive contamination, three types of foods (grains, vegetables, and foods made from multiple ingredients), were sources of almost 83% of the cadmium exposure for children 1-6 years old.
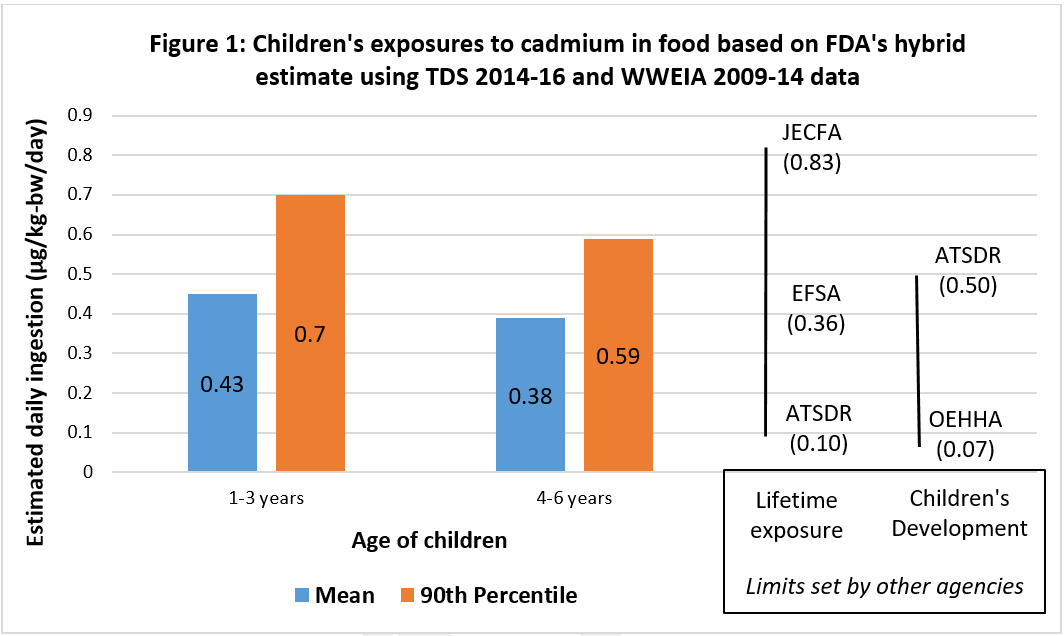
Unlike for lead, FDA has not yet established an MDI for cadmium. Instead, the agency noted three toxicological reference limits for comparison (identified as “Lifetime exposure” in Figure 1 below). These levels were based on the lifetime accumulation of cadmium in the kidneys to levels that would cause harm; the half-life of cadmium in the body is 17 years. In 2011, the Joint FAO/WHO Expert Committee on Food Additives (JECFA), which is a part of the United Nations, set a limit of 0.83 μg/kg-bw/day. The European Food Safety Authority (EFSA) had earlier set a limit of 0.36 μg/kg-bw/day and reaffirmed this value after considering JECFA’s estimate. Separately, CDC’s Agency for Toxic Substances and Disease Registry (ATSDR) set a Minimal Risk Level (MRL) of 0.10 μg/kg-bw/day for chronic oral exposure.
Since FDA is evaluating the emerging evidence of the heavy metal as affecting children’s neurodevelopment, two other reference values (identified as “Children’s development” in Figure 1) may be more relevant to young children:
- In 2012, ATSDR established an MRL of 5 μg/kg-body weight (bw)/day for cadmium to protect children from developmental harm. It was based on effects of cadmium on bone mineral density during the period of rapid skeletal growth in young rats. The exposure via drinking water started at three-weeks of age and lasted 12 months; effects were already observed after three months at the higher intake level.
- In 2001, an expert panel for California’s Office of Environmental Health Hazard Assessment (OEHHA) developed a Maximum Allowable Daily Level (MADL) of 4.1 µg/day or 0.07 µg cadmium/kg-bw/day based on developmental toxicity with safety factors added. The panel relied on a study of pregnant female rats that were exposed to cadmium via drinking water during gestation. The offspring had postnatal development problems such as reduced weight gain and altered locomotor activity.
Figure 1 shows that FDA’s best estimate of mean exposure to cadmium was 0.43 µg/kg-bw/day for 1-3 year-olds, a value that’s very close to the ATSDR MRL for intermediate-duration developmental effects and more than five times the OEHHA MADL. In addition, the 90th percentile for both 1-3 and 4-6 years were over the ATSDR MRL and more than 9 times greater than the OEHHA MADL for the youngest children.
Conclusions
While inorganic arsenic and lead have drawn much of the attention, FDA’s study indicates that cadmium may be as important. Fortunately, the agency is focused on all three as part of its Toxic Elements Workgroup.
FDA’s study shows that a wide variety of foods with low levels of lead or cadmium likely drives exposure. This is in agreement with the agency’s prior statement that “[e]ven though the level of a metal in any particular food is low, our overall exposure adds up because many of the foods we eat contain them in small amounts.” We look forward to seeing FDA translate these total exposures into limits on specific foods, and, based on the newly released data, a cadmium interim reference level based on neurodevelopmental effects.
[1] The TDS program is an ongoing representative sampling of more than 250 types of food sold in the country that are tested for heavy metals, pesticides, nutrients and other chemicals. WWEIA is an ongoing 2-day survey of what the people representative of the US population reported eating on the day before the interview and a second time 3 to 10 days later.
[2] We consider the “hybrid” method developed by FDA to be the best estimate rather than the upper bound and lower bound estimates. In the hybrid method, values less than the limit of detection (LOD) were set to zero if there were no detections for the food type from 2009 to 2016 in the TDS. If there was a detection, the value was set to half of the LOD.