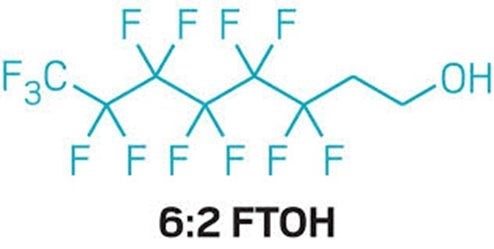
PFAS is a group of synthetic chemicals used in industrial processes and consumer products, including water-repellent clothing, such as outdoor wear, and food packaging. Once these “forever chemicals” are produced and used, they often make their way into the environment and our bodies. Many pose serious threats due to their toxic effects (often at trace levels) and their ability to build up in people, animals and the environment. Studies show that they are in almost all of us.
To make matters worse, people are exposed to multiple PFAS, not individual PFAS in isolation. Yet under the nation’s primary chemical safety law, EPA evaluates the safety of PFAS chemicals one at a time and does not consider the combined risks from exposures to multiple PFAS. Combined exposures increase the risk of harmful effects, thus magnifying the risks and the need for action.
Current Situation: All Costs, No Benefits
PFAS move easily throughout the environment and are difficult to destroy. They have contaminated drinking water, food, farms, wildlife, and the environment more broadly. At the local, state and federal levels, the U.S. is spending millions of dollars to clean up PFAS contamination. Some states, such as Michigan and Maine, are trying to recoup the costs their residents have had to bear to clean-up PFAS contamination of their water and land. The federal government is also taking action to address the widespread PFAS contamination. The costs for cleaning up PFAS contamination are imposed on society by the domestic producers, importers and users of PFAS who profit from their production and use.
Yet, despite the well-documented risks and costs to society of these chemicals, companies still continue to produce, import, and use PFAS. It is time to ban all PFAS or—if there are truly essential uses for these chemicals—limit how they are produced, imported and used so that their impact on us and the environment is minimal.
Urgent Need: Revisit, Reassess, and Regulate All PFAS
While EPA has recently tightened up approvals for new PFAS entering the market, it has yet to take significant action on those that are already on the market, which includes the hundreds of PFAS the agency approved over the past few decades. It is clear these PFAS have not been produced responsibly as demonstrated by the environmental contamination associated with many of the PFAS manufacturing facilities. And yet, many of these PFAS are still on the market. They are being produced and released into the environment, are in products we use every day, and continue to contaminate us and our environment.
Many of EPA’s approvals were made 10 to 20 years ago, before we had a full picture of the pervasiveness and degree of PFAS contamination. The data on the extent of the environmental contamination of these persistent PFAS, their ability to move through the environment, and the significant difficulty in destroying them was not as robust as it is today. Furthermore, mounting evidence shows that even trace levels of PFAS can cause developmental issues in children, reduced fertility, hormonal disruptions, and certain types of cancer.
In addition, these approvals did not consider risks to vulnerable groups, such as pregnant women and children as currently required by the law. Many communities are exposed to multiple PFAS, particularly those who live, work and play near where PFAS are made and used.
Addressing the production, import and use of PFAS would limit further pollution of our water supplies, safeguard the health of our communities, and be consistent with other strong EPA actions to address PFAS, including its recent robust proposed drinking water standards.
Effective regulation of these harmful chemicals at their source would also accelerate efforts to seek out and adopt safer alternatives. Leaving chemicals with such well-documented harms on the market makes it more difficult for innovative, safer substitutes to enter it. Failing to address these risks in effect puts a thumb on the scale in support of older harmful technologies.
Our Take
EPA should re-evaluate each of the PFAS it has approved. During that re-evaluation, EPA should use the best available science and consider the full picture of PFAS exposure. Considering each PFAS in isolation rather than the multiple PFAS people, particularly those in vulnerable groups, are exposed to will underestimate their risk.
EPA should use the Toxic Substances Control Act to take action to ban these legacy PFAS, or restrict them if the uses are truly essential, rather than continuing to allow the production, import and use of these demonstrably harmful “forever chemicals.”
Go Deeper
Learn more about EDF’s concerns about PFAS and read our follow-up blog on how EPA can use TSCA to turn off the PFAS tap.
EPA’s information on PFAS