By Lindsay McCormick, Senior Manager, Safer Chemicals and Roya Alkafaji, Manager, Healthy Communities
What’s New?
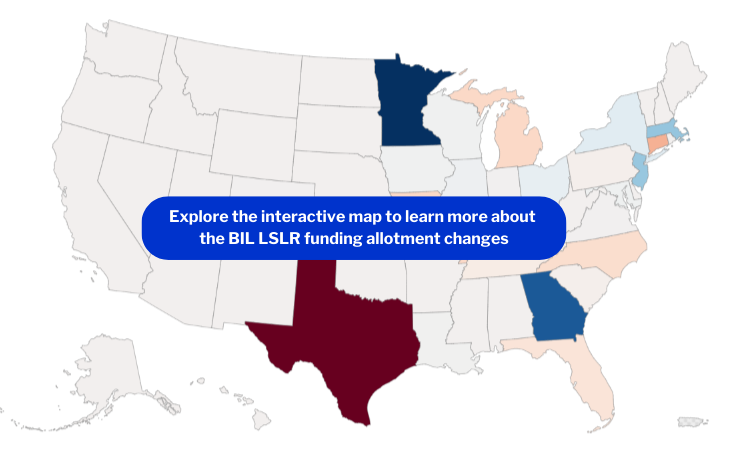
President Biden recently announced $3 billion in federal funding for lead service line replacement. In the third year of this historic $15 billion investment through the Bipartisan Infrastructure Law to replace harmful lead pipes across the U.S., there is an effort to shift where the money is going to better reflect states’ needs. While many saw no or minimal change, others – like Texas, and Minnesota – saw major changes to their funding allotments.
Why It Matters
An estimated 9 million homes and businesses in the U.S. still receive their drinking water through harmful lead pipes. To make the best use of the federal funds aimed at protecting public health, it is critical that the money flows to the states with the greatest need, based on their estimated number of lead service lines without delay.
EPA’s 7th Drinking Water Infrastructure Needs Survey and Assessment (DWINSA), which is the best current estimate of lead service lines across the nation, is the driver for FY23 and FY24 funding allotments. To best reflect the latest information, EPA allowed states and water utilities to submit a “one-time update” to service line information in the fall 2023, thus taking advantage of utilities’ ongoing efforts to inventory their service lines. The goal, which was partially achieved, was to more accurately allocate the funding based on need.
What Changed
EPA reported that 67% of water systems provided a response to the DWINSA update. While we do not know the magnitude of the changes to the state-level lead service line projections as EPA has not yet made this information public *, we do know how it has impacted the funding allotments.
This year, Illinois received the most funding ($241 million), followed by Florida ($229 million), and Ohio ($184 million). Twenty-six states received the minimum allotment of $28.7 million.
Funding allotment changes ranged from minimal to major:
- For 29 states, allotments remained the same or changes were minimal compared with last year.
- Fourteen states received more funding, with Minnesota having the greatest increase (128%).
- Nine received less funding, with Texas having the largest decrease (80%).
We created a map to visualize the most impactful changes from the FY24 funding allotments, compared with FY23.
Our Take
Last year, EDF analyzed the data behind the 7th DWINSA and found flaws in three major areas affecting Texas, New York and Florida. EPA’s fall 2023 one-time update to the DWINSA seems to have corrected two of the three issues we highlighted:
- Texas: Due to an apparent major data entry error in the original survey that inflated the state’s number of lead service lines, Texas received $146 million, the fifth largest allotment, in 2023. This year, the allotment decreased by 80% to $28.7 million. This freed up funds to be distributed to other states with greater need.
- New York: New York saw a 14% increase in its funding this year. New York City, home to over 100,000 lead service lines, did not participate in the original DWINSA, which we suspect dampened the state’s FY23 allotment by drastically underestimating the state’s total lead service lines. New York City’s lead service line estimate is reflected in the fall 2023 one-time update. We do not know with certainty whether New York’s increased FY24 funding can be attributed to New York City’s participation.
- Florida: Florida remained unwavering near the top of the list of states receiving the largest share of funding – over $228 million in FY24. We continue to question whether the state’s allotment matches the true need and are eager to understand how Florida has retained such a large share of the funding without clear evidence of the presence of lead service lines in the state. Large utilities, such as JEA (serving Jacksonville), are just beginning to inventory their lead service lines for the October 2024 Lead and Copper Rule deadline. We’ll keep an eye on the state’s Project Priority List to see how the money is being used.
Next Steps
When EPA released the initial DWINSA results in April 2023, we described how the new formula would positively impact funding allotments for lead service line replacement needs by state. It is now critical that EPA make the formula available for replication, as requested by State Revolving Fund administrators. This step would enable state policymakers and the public to better understand how funding decisions are made. EPA should also promptly release the state-level LSL projections from the one-time update, which drove the recent changes in funding amounts.
EDF will continue to monitor how these resources are allocated to ensure these hard-fought funds go towards ensuring everyone has access to safer drinking water. Water utilities should aggressively pursue funding and start replacing lead service lines now while this historic funding is available.
Go Deeper
Read EPA’s press statement and memo on the funding allotments.
*Updated 5/21/24: Since the publication of this blog, EPA published the state-level service line data from the updated DWINSA.