Richard Denison, Ph.D., is a Lead Senior Scientist.
[UPDATES ADDED 8-6-20: See insertions of bracketed italicized text below.]
[pullquote]So much for the Trump EPA’s constantly ballyhooed commitment to transparency under TSCA.[/pullquote]I blogged a few short weeks ago about just how brazen EPA officials have become in aligning themselves with the chemical industry when it comes to the agency’s review of companies’ requests to commercialize new chemicals under the Toxic Substances Control Act (TSCA).
Yet it just keeps getting worse.
Thanks to an article in Inside EPA by a reporter who came across a buried addition to EPA’s new chemicals web pages, we learned that EPA had quietly “updated” the boilerplate language it uses when it negotiates a consent order with a company that sets the terms for market entry of a new chemical. As I have described before, those orders are increasingly rarely issued at all by EPA, with the great majority of chemicals cleared for market entry absent any conditions at all.
Without any announcement or notice, EPA posted the new language here on April 21. EPA states: “The updated version of the boilerplate is expected to be used for Orders starting in April 2020.”
I had heard quite some time ago that revisions to the order language were being considered, with some in industry complaining that the language EPA was putting in the orders was too adversarial and legalistic – that is, too much like, well, an order.
In my earlier blog post, I cited comments by Dr. Lynn Dekleva, EPA’s associate deputy assistant administrator for new chemicals, who came directly to EPA from DuPont where she was responsible for getting the company’s new chemicals approved by EPA. One of Dekleva’s first assignments upon arriving at EPA was to “streamline” the consent order language. The fruits of her labor appear to be what the agency quietly posted in April and now says it’s using.
The new language is indeed heavily streamlined, eliminating important detail that provides, for example, for enforceability – a concern that EPA’s own Inspector General raised lust last month in the context of TSCA consent orders issued even before the new changes.
EPA quietly vetted the new language with industry
As also reported by Inside EPA, the agency took it upon itself to “informally” vet the new language with industry representatives, to the exclusion of other stakeholders. You’d almost think the agency has forgotten that the industry it is supposed to regulate is not its “client.”
In response to these disturbing disclosures, EDF, Safer Chemicals Healthy Families and several other NGOs sent a letter last Friday to Alexandra Dunn, head of EPA’s Office of Chemical Safety and Pollution Prevention, calling on EPA “to suspend use of the new order, explain how it changes the old order and why these changes were made, and afford an opportunity for public comment.”
So where are the consent orders using the new language?
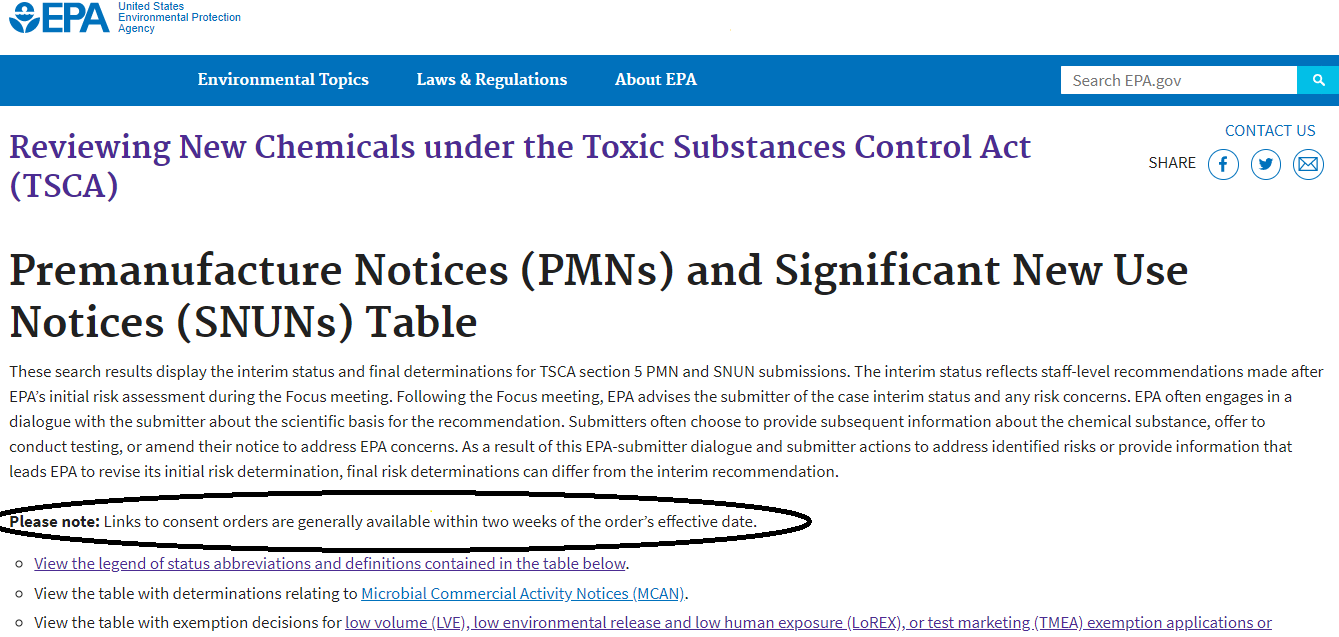
Given EPA’s statement that it expected to begin using the new boilerplate language in April, I went to look at some recent consent orders to see if they used the new language. Links to the orders are supposed to be provided in two places: On the “status table” listing EPA’s decisions on new chemicals; and in ChemView, the agency’s repository for information it receives and decision documents it issues under TSCA. Here’s what I found (or more accurately, did not find):
Status table: On the status table EPA notes that it generally provides a link to a consent order within two weeks of its effective date; see the circled text below:
[UPDATE ADDED 8-6-20: After our blog post, EPA changed the text on this webpage to remove the reference to making consent orders generally available within two weeks. The language nows says: “Please note: Access to documents relating to TSCA Section 5 Actions is available at regulations.gov.” This link is utterly useless: It goes to the top page of a site that applies to regulations across the entire Federal government, making it all but impossible for a user to actually locate consent orders or any other documents relating to new chemicals under TSCA.]
The status table lists a number of premanufacture notices (PMNs) subject to final consent orders in recent months. But the most recent such listing in this table that has an actual link to the order itself is for one with an effective date of well over a year ago. This was the consent order for a group of PMNs – numbered P-16-0151 through 0155 – that became effective on May 8, 2019.
ChemView: When provided, the links in the status table take the user to the consent orders themselves in ChemView, so I looked there to see if any more recent ones were available. The most recent order posted there was one covering two PMNs that had an effective date in April of this year, but a number of additional PMNs that EPA’s status table indicates have final orders with effective dates in April as well as May – all of them much longer ago than two weeks – were not accessible. So, not only are these orders not linked to from the status table, they aren’t available through ChemView either.
I then inquired with EPA as to why these consent orders are not being made available to the public. I was told that EPA stopped posting orders to ChemView in April, due to “funding issues,” and that EPA expected this situation would continue, i.e., no orders would be posted to ChemView or links added to the status table, at least through the end of the fiscal year (September 30).
[UPDATE ADDED 8-6-20: As of this date EPA is still not posting new consent orders to ChemView.]
This timing is troubling: Just as EPA started using its “streamlined” language in the specific consent orders it is negotiating with companies, EPA has rendered it impossible for the public to access those orders. So much for the Trump EPA’s constantly ballyhooed commitment to transparency under TSCA.
Moreover, it appears that when EPA’s TSCA office experiences “funding issues,” the first thing to go are services that benefit the public interest and shed light on EPA decisions. There appears to be no such cutback or slowdown in EPA’s relentless effort to speed up new chemical approvals, which my last blog post made clear the Trump EPA considers job number one.
EPA’s lame excuse for not publicly vetting the new language
In response to the letter we sent to EPA, Inside EPA reports that EPA responded as follows (emphasis added): “Per EPA’s customary approach to developing orders under our regulatory programs, the agency did not solicit public comments on the updated version.”
Yet EPA did solicit industry comments. This is the crux of the problem: EPA’s mindset is that the new chemicals program is purely bilateral between it and the chemical industry, with no room for the public (or workers or affected communities), and that mindset is now on steroids under the Trump administration. While this is a longstanding problem, the changes made to the new chemicals provisions of TSCA through the 2016 reforms were intended to address this.
This episode reveals that this EPA, especially its conflicted political hires and appointees, think nothing of – and think there is nothing wrong with – allowing or inviting the industry into the room while locking out the public. Numerous times over the last several years it has come out that policy or decision documents on new chemicals were shared with companies to get their “informal feedback” with no public notice it was even being done, let alone an opportunity to provide feedback. See here and here. This new episode is only the latest example.
Lastly, this excuse is particularly rich, coming from an EPA that claims it is all about transparency and the need for public vetting when it comes to any sort of guidance document EPA uses.