By Maricel Maffini, PhD, Consultant, and Tom Neltner, JD
What Happened
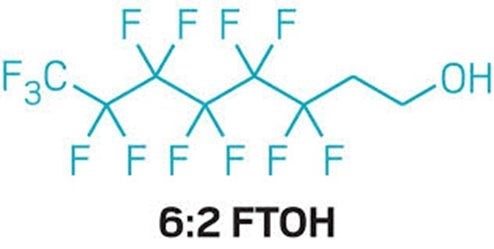
In December 2023, FDA’s scientists published a new study showing that when pregnant rats ingest a form of per- and polyfluorinated alkyl substance (PFAS) called 6:2 fluorotelomer alcohol (6:2 FTOH) their bodies break it down into other PFAS that reach the fetuses and biopersist in the mother and the pups.
The study also showed that the body of a non-pregnant animal produces different breakdown products that also biopersist. This study is the latest evidence that the assumptions made about the safety of short-chain PFAS (chemicals with fewer than 8 carbons) have been wrong. Read More