Samantha Lovell is a Project Specialist. Lindsay McCormick, is a Project Manager.
Today, families from across the country came to Washington, DC to tell lawmakers how the toxic chemical trichloroethylene (TCE) has impacted their lives.
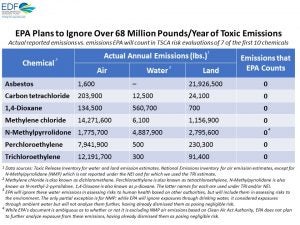
TCE is a known human carcinogen that is toxic to the immune system and kidneys, and can cause fetal heart damage – among other harmful health effects. The Environmental Protection Agency (EPA) proposed bans on high-risk uses of TCE under the newly reformed Toxic Substances Control Act (TSCA) back in December 2016 and January 2017, but under this Administration, the agency has abandoned these bans.
TCE is also one of the first 10 chemicals slated for a broad risk review by EPA under TSCA. Unfortunately, EPA plans to ignore the major exposures Americans face from TCE and other toxic chemicals released to our air, water and land – yet another sign that EPA is giving in to the chemical industry to the detriment of the public’s health.
In a moving press conference today led by Sen. Tom Udall, several families shared their stories in an effort to pressure EPA to finalize the bans and take other necessary steps to protect communities across the country from TCE. Read More