PART 1: EPA rams through its reckless review scheme for new chemicals under TSCA, your health be damned
Richard Denison, Ph.D., is a Lead Senior Scientist.
Overruling the recommendations of its own longtime professional staff, political appointees at EPA have begun green-lighting new chemicals to enter commerce using an approach that shows contempt for the letter and intent of the 2016 reforms to the Toxic Substances Control Act (TSCA).
EDF blogged recently about the new approach and how it drastically deviates from what the law requires.[pullquote]Now any company will be free to produce, import and use the chemical in any manner it chooses and without any obligation to inform EPA of its activities.[/pullquote]
Today EPA posted the first decision made under the new scheme: It issued a “not likely to present an unreasonable risk” determination for a chemical that, according to its manufacturer International Flavors and Fragrances Inc., is to be imported for use “to reduce malodors. It will be sold to industrial and commercial customers for their incorporation into industrial, commercial, and household consumer products such as floor cleaners, cat litters, fabric refresher sprays, Etc.”
The “not likely” finding means that International Flavors and Fragrances Inc. can commence manufacture and sale of the chemical, and will not be subject to any conditions or limits. Once manufacture starts, the chemical will be placed on the TSCA Inventory, and that company or any other will be free to produce, import and use the chemical in any manner it chooses and without any obligation to inform EPA of its activities.
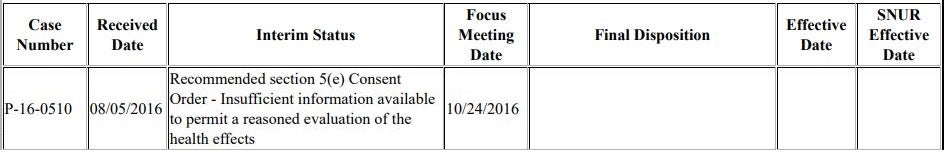
The company submitted a premanufacture notice (PMN) for the chemical in August 2016 and EPA assigned it the number P-16-0510. Based on its original review of the PMN, EPA’s staff made this initial recommendation:
EPA’s decision today is a dramatic departure from that initial recommendation. Based on the new approach EPA is taking, not only will no consent order be developed, but EPA will also not pursue promulgation of a Significant New Use Rule (SNUR) for the chemical. Consent orders and SNURs are the two means by which EPA can place limits or require testing or more information on a new chemical. EPA is forgoing those options.
Unlike the great majority of new chemicals submitted under TSCA, however, in this case the identity of the chemical was disclosed. The chemical and its Chemical Abstract Service (CAS) number are as follows:
Oxirane, 2-methyl-, polymer with oxirane, bis[2-[(1-oxo-2-propen-1-yl)amino]propyl] ether
CAS 1792208-65-1
EPA has established a set of “chemical categories” it uses to flag new chemicals that could pose serious health or environmental concerns without conditions placed on their production or use. EPA’s determination document notes that this chemical falls into its “acrylamides” category. This likely reveals why EPA staff raised concerns from the start. Here is EPA’s description of the hazard concerns for this category:
Based on analogy to acrylamide per se, members of the class are considered potential carcinogens, heritable mutagens, reproductive and developmental toxicants, and toxic to aquatic organisms. Acrylamides are also potential neurotoxins based on data for a number of low molecular weight acrylamides.
In its own determination document, EPA identifies these toxicities and more, noting “the potential for the following human health hazards: irritation, mutagenicity, developmental/reproductive toxicity, neurotoxicity, and carcinogenicity.”
So, using its new scheme, it appears that the first chemical EPA has cleared to enter commerce without any restrictions or testing to address the health concerns whatsoever is one that:
- has clear exposure potential as evidenced by its intended dispersive use in various consumer products;
- may pose serious hazards; and
- was flagged by EPA staff as having serious information gaps and raising enough concern to recommend some degree of restriction and, likely, testing.
That certainly doesn’t sound like a chemical that EPA should allow to be produced and used in any amount and for any use that any company desires, but that’s what EPA has done.
And this is only the beginning: We fear many more such decisions will follow shortly.
But this much is clear: The new trajectory EPA is on will not only further erode the public’s confidence in our chemical safety system. It may well put the public’s health at greater risk than even under the old TSCA.