Richard Denison, Ph.D., is a Lead Senior Scientist.
[pullquote]The intentional alteration of industry study summaries under REACH that I report here should raise major alarms on both sides of the Atlantic and illustrates why public access to full studies on chemicals to which we are or may be exposed must be paramount.[/pullquote]Well, I certainly wasn’t expecting to find this when I started working on EDF’s comments on supplemental materials the Environmental Protection Agency (EPA) recently made available on Pigment Violet 29 (PV29), the first of 10 chemicals undergoing risk evaluations under the amended Toxic Substances Control Act (TSCA). What I discovered – almost by chance – strongly reinforces EDF’s and others’ view that the public’s ability to independently assess and trust EPA chemical assessments falls flat without access to full and unredacted copies of the health and safety information EPA cites in support of its risk evaluation.
When EPA first released a draft of its PV29 risk evaluation for comment, it refused to make public the underlying health and safety studies. Instead, it only provided copies of and links to brief summaries – misleadingly called “robust summaries” – of the studies, which were prepared by companies that make PV29. The companies had submitted the summaries to the European Chemicals Agency (ECHA) when they registered PV29 under the European Union’s REACH Regulation.
EPA first claimed the studies had to be withheld because they were protected as confidential business information (CBI) under TSCA – a position that is flatly contradicted by the plain text of the law. In response to the outcry that ensued, EPA recently made public more of the content of these health and safety studies. Unfortunately, EPA is still withholding key parts of that body of information under a different, equally flawed theory it has advanced to allow companies to hide health and safety information that TSCA requires be made public. For more detail, see this recent letter sent to EPA by groups, including EDF, who sought to obtain the studies through a Freedom of Information Act (FOIA) request that EPA now has denied with respect to the full release of these studies. Stay tuned on that.
Meanwhile, EPA has reopened the comment period for the PV29 risk evaluation to allow stakeholders to comment anew on the additional, but still partial, information it released. As I was reviewing EDF’s prior comments in preparing our new ones, I discovered that some of the “robust summaries” EPA placed in its PV29 docket back in November have changed: The current versions of at least two of those summaries now posted on ECHA’s REACH registration website show deletions of certain information that was in the versions EPA first made available.
I noticed the changes because the deleted information is the same information EDF had cited in our earlier comments as undermining a key conclusion EPA had drawn about PV29. When I went to ECHA’s website to refresh my memory of those summaries, I sensed something had changed and, sure enough, by comparing the original and current versions of two of the summaries, I soon detected that someone has made small but significant deletions.
Below I describe and document the changes I found. But before dragging you through that, consider what this means.
Implications for public trust in EPA assessments
The Trump EPA and the chemical industry have been arguing that the public can and should make do with the “robust summaries” rather than have access to the full study reports that TSCA requires be made public. In its draft risk evaluation, EPA emphasized that the summaries could be trusted because EPA had verified that they accurately reflected the underlying studies; in short, we were told we could and should take their word for it.
Now with this new development, we have learned two new things:
- First, these summaries can be altered at will, without any apparent notice or explanation; in this case, we suspect the changes were made in direct reaction to EDF’s comments citing the now-deleted information.
- Second, because of these changes, the full study reports are NOT, as EPA claimed in its draft risk evaluation, “consistent with the physical and chemical characteristics … as presented in the ECHA robust summaries.”
As explained below, values for a key physical-chemical characteristic of PV29 have vanished from the study summaries now posted on ECHA’s website. We know of this inconsistency because EPA, as part of its recent release, has finally made public the full study reports (one of them with some redactions) that correspond to the two altered summaries. The values for that key PV29 characteristic deleted from the summaries are still in the full study reports (see below); presumably, those full reports (which date back to 1988 and 1999) would be much more difficult to alter without drawing attention.
Add this to the long list of reasons why it is wholly unacceptable to expect the public to rely on summaries prepared by the companies making a chemical under review, or to trust EPA’s assertion that the summaries accurately reflect the underlying studies.
Implications for REACH
A sidebar here seems warranted. Someone appears to have entered the ECHA registration data system and made these changes without leaving any public trace of having done so. The altered summaries display no date or other indication that they have been altered.
This begs some questions of ECHA and the EU member state authorities that implement the EU’s REACH Regulation:
- Do the authorities have any way of knowing when alterations are made to registration information submitted by a company?
- Can they tell what changes were made and who made the changes?
- Can a change be made without any explanation or justification for the change being provided?
- Can companies simply exclude from or later alter information in its “robust summaries”at will? Given that this summary information is public but the underlying studies are not made public under REACH, shouldn’t the alteration of this information at least be flagged to the public?
- If changes are made to REACH registration information after various checks have been performed on the information, shouldn’t those later alterations raise red flags?
- Do checks of summaries ensure that they faithfully report the information contained in the underlying study, or do the companies get to pick-and-choose what information to include in a summary?
- Doesn’t this situation indicate the need for full studies to be available to other governments that seek to rely on data submitted under REACH, as well as the public?
Here’s what was altered
In EDF’s earlier comments on PV29, we questioned EPA’s reliance on a single value – 0.01 milligrams per liter (mg/L) – to assert that PV29’s water solubility is very low, and then its repeated reliance on that property to dismiss a host of other concerns. Among our arguments was that the summaries of two other studies EPA cited indicated much higher values for PV29’s water solubility:
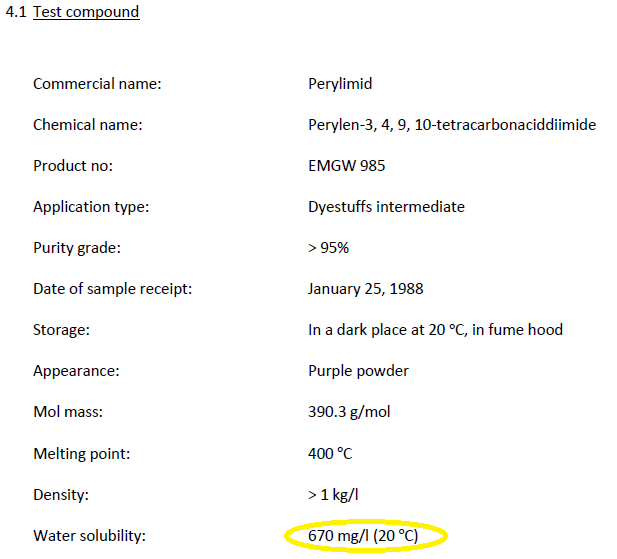
- Study 1: The summary of a 1988 study that reported testing of PV29’s acute toxicity to freshwater fish, which used OECD Test Guideline #203 and indicated it complied with Good Laboratory Practices (GLP), listed the chemical’s water solubility as 670 mg/L – 67,000 times higher than the value EPA relies on.
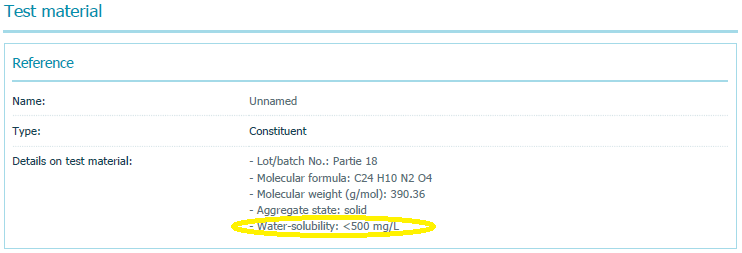
- Study 2: The summary of a 1999 study measuring PV29’s biodegradability, which used OECD Test Guideline #301F and was GLP-compliant, reported the chemical’s water solubility as “<500 mg/L,” or up to 50,000 times higher than the value EPA relies on.
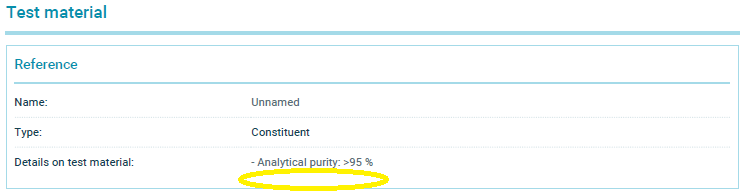
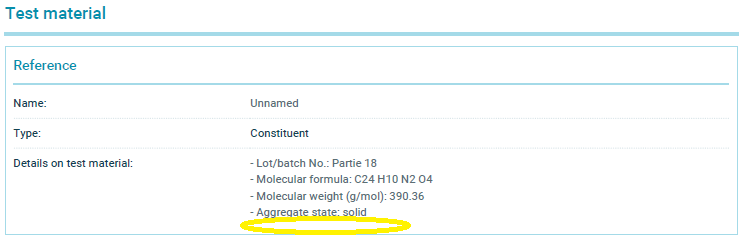
These two summaries have now been selectively altered: The only change made to each is to simply delete the water solubility values of 670 mg/L and <500 mg/L that we had cited.
The summary of Study 1 that EPA printed from the ECHA website on November 13, 2018, and posted to its PV29 docket is available here. And here is a screen shot of the relevant section of that summary where I have circled the water solubility value:
The summary of Study 1 that I printed from the ECHA website today is available here. And here is a screen shot of the relevant section of that current summary where I have circled where the water solubility value has been deleted:
The summary of Study 2 that EPA printed from the ECHA website on November 13, 2018, and posted to its PV29 docket is available here. And here is a screen shot of the relevant section of that summary where I have circled the water solubility value:
The summary of Study 2 that I printed from the ECHA website today is available here. And here is a screen shot of the relevant section of that current summary where I have circled where the water solubility value has been deleted:
I noted above that EPA has now made full study reports (one with some redactions) publicly available. As I also noted, the values now missing from the current study summaries are in the full study reports.
Study 1 is available here. And here is a screen shot from page 8 of that study report showing the value that was in the original summary but has now been deleted from the currently posted summary:
Study 2 is available here. And here is a screen shot from page 7 of that study report showing the value that was in the original summary but has now been deleted from the currently posted summary:
Conclusion
Despite the clear expression of Congressional intent in TSCA – dating back to the original law enacted in 1976 – that health and safety studies and associated information on chemicals should be public, the chemical industry and its allies now embedded within EPA continue to seek ways to circumvent that intent. The case of PV29 is Exhibit A, illustrating ongoing efforts to withhold such studies or provide versions with critical data redacted, and a continuing insistence that the public should make do with industry-prepared summaries of the studies. The intentional alteration of those summaries that I report here should raise major alarms on both sides of the Atlantic and illustrates why public access to full studies on chemicals to which we are or may be exposed must be paramount. I only hope it does.










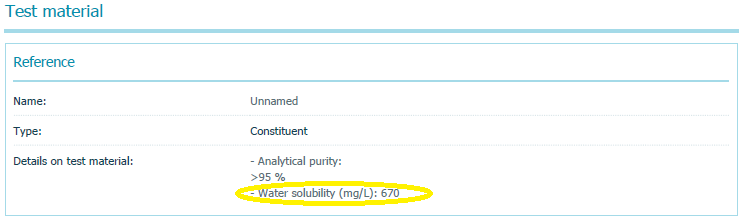

5 Comments
Dear Dr Denison,
Thank you for your interest in data generated under EU’s chemicals regulation REACH and for your questions to ECHA.
Under REACH, industry is responsible for generating the data based on which the safe use can be determined, and to report them in the REACH registration dossier.
The only source of information for the data on ECHA’s website is these registration dossiers submitted by companies who manufacture or import the substance to EU. The publicly available data changes automatically if a registrant makes changes to their registration dossier. ECHA encourages companies to proactively keep their registrations up to date. REACH requires that registrants submit all available information on their substances.
All REACH registration dossiers undergo a completeness check before they are published on ECHA’s website. This process does not verify the quality and adequacy of the data. These aspects are verified during an assessment of the dossier, which is carried out during the evaluation process under REACH.
In this particular case, ECHA is currently processing the decision making of a dossier evaluation draft decision. The registration dossier has recently been updated following ECHA’s request.
Although ECHA’s website only shows the latest version of REACH registration dossiers, ECHA has at its disposal all versions submitted since the initial submission. Therefore we can compare versions to verify whether data has been amended. When assessing the risks and hazards of any substance, the Agency can also request access to the full studies.
ECHA is committed to continue increasing transparency on the data on substances, including how to indicate any changes made to registration dossiers on our website.
Best regards
Christel Musset
Director of Hazard Assessment
European Chemicals Agency (ECHA)
https://echa.europa.eu/
Dear Ms. Musset:
Thank you so much for taking the time to respond to my blog post. It is very helpful that you clarify that the dossiers are posted by companies and have not been reviewed by ECHA for quality or adequacy of the data. In its draft risk evaluation for PV29, EPA erroneously stated that the dossiers were prepared by ECHA.
I also appreciate your noting that ECHA is considering “how to indicate any changes made to registration dossiers on our website.” This is a central concern I have raised, as the public does not have access to the multiple versions that may exist of a company’s study summary and has no way to know whether a change has been made, what changed – and equally important – the reason for the change and why it is warranted.
Your response does lead me to pose some additional questions:
• You note that ECHA requested the dossier be updated. I assume, however, that ECHA did not request the company(ies) to delete the specific water solubility values that were removed from the study summaries I flagged in my blog post? Is that correct?
• You note that ECHA has access to prior versions of a company’s study summary. Can ECHA readily see precisely what has changed from one version to the next, as opposed to having to painstakingly compare the latest version to prior ones?
• Are companies required or expected to explain why a change has been made, or can changes such as those I identify be made without providing any explanation/justification to ECHA?
• As part of the “decision making of a dossier evaluation draft decision” that you indicate is underway, will ECHA examine the various versions of study summaries that a company has posted to see what changes were made and why?
• Does the completeness check you describe include a determination as to whether a summary adequately describes the underlying study? If so, does ECHA address whether that determination of completeness needs to be revisited if there are subsequent changes made to the study summaries?
• Does ECHA have access to the full study reports for the studies summarized in the dossier for this chemical? If not, will it obtain them?
• Does ECHA know how often changes of the sort I flagged are made by companies to their dossiers? Is what I have described an exceptional circumstance or common?
I believe the issues we are discussing are very important and have global significance, given the prominent role REACH is playing globally and the use of the data ECHA is collecting by governments in countries outside the EU. I would welcome more dialogue on these issues.
Best regards,
Richard Denison
Dear Richard Denison
Thank you for your follow-up questions.
One opportunity for an open face-to-face dialogue on this type of questions would be at ECHA’s annual conference, which will take place in Helsinki on 21-22 May 2019 and is also web streamed. The event is free of charge. Should you be interested in joining the ECHA Conference, you can find more information here: https://echa.europa.eu/-/safer-chemicals-2019.
Our conference will be held back-to-back with the Helsinki Chemicals Forum (23-24 May 2019) and Setac Europe (26-30 May 2019).
Regarding your questions, please find some short answers below:
1. You note that ECHA requested the dossier be updated. I assume, however, that ECHA did not request the company(ies) to delete the specific water solubility values that were removed from the study summaries I flagged in my blog post? Is that correct?
ECHA’s evaluation decisions are published on the Agency’s website: https://echa.europa.eu/information-on-chemicals/dossier-evaluation-status .
The evaluation decisions concerning PV29 (EC 201-344-6) will also be published there in due course. ECHA has naturally not requested any data to be deleted from the registration dossier.
2. You note that ECHA has access to prior versions of a company’s study summary. Can ECHA readily see precisely what has changed from one version to the next, as opposed to having to painstakingly compare the latest version to prior ones?
We have tools for readily verifying what has changed across versions of a dossier. This verification is conducted when a dossier is being assessed, e.g. for a compliance check. We are working on how these tools can be further developed to build this as a standardised process during the submission of the information and to make it more transparent on our website.
Note that our registration database contains ca. 90 000 dossiers and that we are receiving many registrations (ca. 15 000 dossiers) on a yearly basis, either as initial dossiers or updates.
3. Are companies required or expected to explain why a change has been made, or can changes such as those I identify be made without providing any explanation/justification to ECHA?
If changes are done further to an evaluation decision (compliance check or examination of testing proposals by ECHA, or substance evaluation by the EU Member States), companies are providing scientific justifications for the changes done on the information requested.
But dossiers are updated continually for many reasons: the REACH Regulation requires registrants to update their dossiers without any delay for any change in their status, the composition of their substances, their uses, the tonnages manufactured or imported, new knowledge on potential risks, classification, chemical safety report, etc.
Although information should not be deleted, it is however possible that certain outcomes of risk assessment could be changed due to new information (e.g. new experimental data obtained through testing) at the disposal of the registrants.
4. As part of the “decision making of a dossier evaluation draft decision” that you indicate is underway, will ECHA examine the various versions of study summaries that a company has posted to see what changes were made and why?
We will verify what has changed. However, in the case of the substance in question, the information requests of the current draft decision were on the human health part of the dossier. The endpoints covering the two study summaries, which included the water solubility details, were not assessed at this stage.
5. Does the completeness check you describe include a determination as to whether a summary adequately describes the underlying study? If so, does ECHA address whether that determination of completeness needs to be revisited if there are subsequent changes made to the study summaries?
As described above, ECHA receives many dossiers. Registration dossiers are only part of this. We also receive notifications for classification and labelling (over 6.5 million so far), applications for authorisation, inquiries for new substances, biocidal dossiers, etc.
The completeness check entails automated verifications and a manual verification for ca. 30 % of the registration dossiers. This is to verify that all information requested under REACH is duly submitted. During this process, there is no assessment of the information or comparison with previous versions of a dossier.
Under REACH, registrants are however required to submit all available information on their substances. As regards whether the information has changed compared to a previous version, this is assessed during the compliance check under dossier evaluation.
6. Does ECHA have access to the full study reports for the studies summarized in the dossier for this chemical? If not, will it obtain them?
ECHA’s risk and hazard assessment is based on robust study summaries provided by companies in their REACH registration dossiers. But ECHA can ask for an access to full studies and raw data, if necessary.
7. Does ECHA know how often changes of the sort I flagged are made by companies to their dossiers? Is what I have described an exceptional circumstance or common?
We do not have this information. ECHA encourages companies to keep their registration dossier up to date, reflecting the latest knowledge on the intrinsic properties and use of the substances with a focus on adding any new information available or editing information that may change like tonnages or uses.
Best regards,
Christel Musset
Director of Hazard Assessment
European Chemicals Agency (ECHA)
Dear Richard Denison
Thank you for your follow-up questions.
One opportunity for an open face-to-face dialogue on this type of questions would be at ECHA’s annual conference, which will take place in Helsinki on 21-22 May 2019 and is also web streamed. The event is free of charge.
Our conference will be held back-to-back with the Helsinki Chemicals Forum (23-24 May 2019) and Setac Europe (26-30 May 2019).
Regarding your questions, please find some short answers below:
1. You note that ECHA requested the dossier be updated. I assume, however, that ECHA did not request the company(ies) to delete the specific water solubility values that were removed from the study summaries I flagged in my blog post? Is that correct?
ECHA’s evaluation decisions are published on the Agency’s website.
The evaluation decisions concerning PV29 (EC 201-344-6) will also be published there in due course. ECHA has naturally not requested any data to be deleted from the registration dossier.
2. You note that ECHA has access to prior versions of a company’s study summary. Can ECHA readily see precisely what has changed from one version to the next, as opposed to having to painstakingly compare the latest version to prior ones?
We have tools for readily verifying what has changed across versions of a dossier. This verification is conducted when a dossier is being assessed, e.g. for a compliance check. We are working on how these tools can be further developed to build this as a standardised process during the submission of the information and to make it more transparent on our website.
Note that our registration database contains ca. 90 000 dossiers and that we are receiving many registrations (ca. 15 000 dossiers) on a yearly basis, either as initial dossiers or updates.
3. Are companies required or expected to explain why a change has been made, or can changes such as those I identify be made without providing any explanation/justification to ECHA?
If changes are done further to an evaluation decision (compliance check or examination of testing proposals by ECHA, or substance evaluation by the EU Member States), companies are providing scientific justifications for the changes done on the information requested.
But dossiers are updated continually for many reasons: the REACH Regulation requires registrants to update their dossiers without any delay for any change in their status, the composition of their substances, their uses, the tonnages manufactured or imported, new knowledge on potential risks, classification, chemical safety report, etc.
Although information should not be deleted, it is however possible that certain outcomes of risk assessment could be changed due to new information (e.g. new experimental data obtained through testing) at the disposal of the registrants.
4. As part of the “decision making of a dossier evaluation draft decision” that you indicate is underway, will ECHA examine the various versions of study summaries that a company has posted to see what changes were made and why?
We will verify what has changed. However, in the case of the substance in question, the information requests of the current draft decision were on the human health part of the dossier. The endpoints covering the two study summaries, which included the water solubility details, were not assessed at this stage.
5. Does the completeness check you describe include a determination as to whether a summary adequately describes the underlying study? If so, does ECHA address whether that determination of completeness needs to be revisited if there are subsequent changes made to the study summaries?
As described above, ECHA receives many dossiers. Registration dossiers are only part of this. We also receive notifications for classification and labelling (over 6.5 million so far), applications for authorisation, inquiries for new substances, biocidal dossiers, etc.
The completeness check entails automated verifications and a manual verification for ca. 30 % of the registration dossiers. This is to verify that all information requested under REACH is duly submitted. During this process, there is no assessment of the information or comparison with previous versions of a dossier.
Under REACH, registrants are however required to submit all available information on their substances. As regards whether the information has changed compared to a previous version, this is assessed during the compliance check under dossier evaluation.
6. Does ECHA have access to the full study reports for the studies summarized in the dossier for this chemical? If not, will it obtain them?
ECHA’s risk and hazard assessment is based on robust study summaries provided by companies in their REACH registration dossiers. But ECHA can ask for an access to full studies and raw data, if necessary.
7. Does ECHA know how often changes of the sort I flagged are made by companies to their dossiers? Is what I have described an exceptional circumstance or common?
We do not have this information. ECHA encourages companies to keep their registration dossier up to date, reflecting the latest knowledge on the intrinsic properties and use of the substances with a focus on adding any new information available or editing information that may change like tonnages or uses.
Best regards,
Christel Musset
Director of Hazard Assessment
European Chemicals Agency (ECHA)
Dear Richard Denison
Thank you for your follow-up questions.
One opportunity for an open face-to-face dialogue on this type of questions would be at ECHA’s annual conference, which will take place in Helsinki on 21-22 May 2019 and is also web streamed. The event is free of charge. Should you be interested in joining the ECHA Conference, you can find more information here: https://echa.europa.eu/-/safer-chemicals-2019.
Our conference will be held back-to-back with the Helsinki Chemicals Forum (23-24 May 2019) and Setac Europe (26-30 May 2019).
Regarding your questions, please find some short answers below:
1. You note that ECHA requested the dossier be updated. I assume, however, that ECHA did not request the company(ies) to delete the specific water solubility values that were removed from the study summaries I flagged in my blog post? Is that correct?
ECHA’s evaluation decisions are published on the Agency’s website: https://echa.europa.eu/information-on-chemicals/dossier-evaluation-status .
The evaluation decisions concerning PV29 (EC 201-344-6) will also be published there in due course. ECHA has naturally not requested any data to be deleted from the registration dossier.
2. You note that ECHA has access to prior versions of a company’s study summary. Can ECHA readily see precisely what has changed from one version to the next, as opposed to having to painstakingly compare the latest version to prior ones?
We have tools for readily verifying what has changed across versions of a dossier. This verification is conducted when a dossier is being assessed, e.g. for a compliance check. We are working on how these tools can be further developed to build this as a standardised process during the submission of the information and to make it more transparent on our website.
Note that our registration database contains ca. 90 000 dossiers and that we are receiving many registrations (ca. 15 000 dossiers) on a yearly basis, either as initial dossiers or updates.
3. Are companies required or expected to explain why a change has been made, or can changes such as those I identify be made without providing any explanation/justification to ECHA?
If changes are done further to an evaluation decision (compliance check or examination of testing proposals by ECHA, or substance evaluation by the EU Member States), companies are providing scientific justifications for the changes done on the information requested.
But dossiers are updated continually for many reasons: the REACH Regulation requires registrants to update their dossiers without any delay for any change in their status, the composition of their substances, their uses, the tonnages manufactured or imported, new knowledge on potential risks, classification, chemical safety report, etc.
Although information should not be deleted, it is however possible that certain outcomes of risk assessment could be changed due to new information (e.g. new experimental data obtained through testing) at the disposal of the registrants.
4. As part of the “decision making of a dossier evaluation draft decision” that you indicate is underway, will ECHA examine the various versions of study summaries that a company has posted to see what changes were made and why?
We will verify what has changed. However, in the case of the substance in question, the information requests of the current draft decision were on the human health part of the dossier. The endpoints covering the two study summaries, which included the water solubility details, were not assessed at this stage.
5. Does the completeness check you describe include a determination as to whether a summary adequately describes the underlying study? If so, does ECHA address whether that determination of completeness needs to be revisited if there are subsequent changes made to the study summaries?
As described above, ECHA receives many dossiers. Registration dossiers are only part of this. We also receive notifications for classification and labelling (over 6.5 million so far), applications for authorisation, inquiries for new substances, biocidal dossiers, etc.
The completeness check entails automated verifications and a manual verification for ca. 30 % of the registration dossiers. This is to verify that all information requested under REACH is duly submitted. During this process, there is no assessment of the information or comparison with previous versions of a dossier.
Under REACH, registrants are however required to submit all available information on their substances. As regards whether the information has changed compared to a previous version, this is assessed during the compliance check under dossier evaluation.
6. Does ECHA have access to the full study reports for the studies summarized in the dossier for this chemical? If not, will it obtain them?
ECHA’s risk and hazard assessment is based on robust study summaries provided by companies in their REACH registration dossiers. But ECHA can ask for an access to full studies and raw data, if necessary.
7. Does ECHA know how often changes of the sort I flagged are made by companies to their dossiers? Is what I have described an exceptional circumstance or common?
We do not have this information. ECHA encourages companies to keep their registration dossier up to date, reflecting the latest knowledge on the intrinsic properties and use of the substances with a focus on adding any new information available or editing information that may change like tonnages or uses.
Best regards,
Christel Musset
Director of Hazard Assessment
European Chemicals Agency (ECHA)
https://echa.europa.eu/