By Tom Neltner, Senior Director, Safer Chemicals, Klara Matouskova, PhD, Consultant, and Maricel Maffini, PhD, Consultant
What Happened?
In our new study, we evaluated Generally Recognized As Safe (GRAS) notices—a total of 403 between 2015-2020—that food manufacturers voluntarily submitted to FDA for review. Our goal was to determine whether industry was adhering to FDA’s Guidance on Best Practices for Convening a GRAS Panel.
The guidance was designed to help companies comply with the law and avoid biases and conflicts of interest when determining whether substances added to food are safe and recognized as such by the scientific community. FDA published a draft of the guidance in 2017 and finalized it essentially unchanged in December 2022.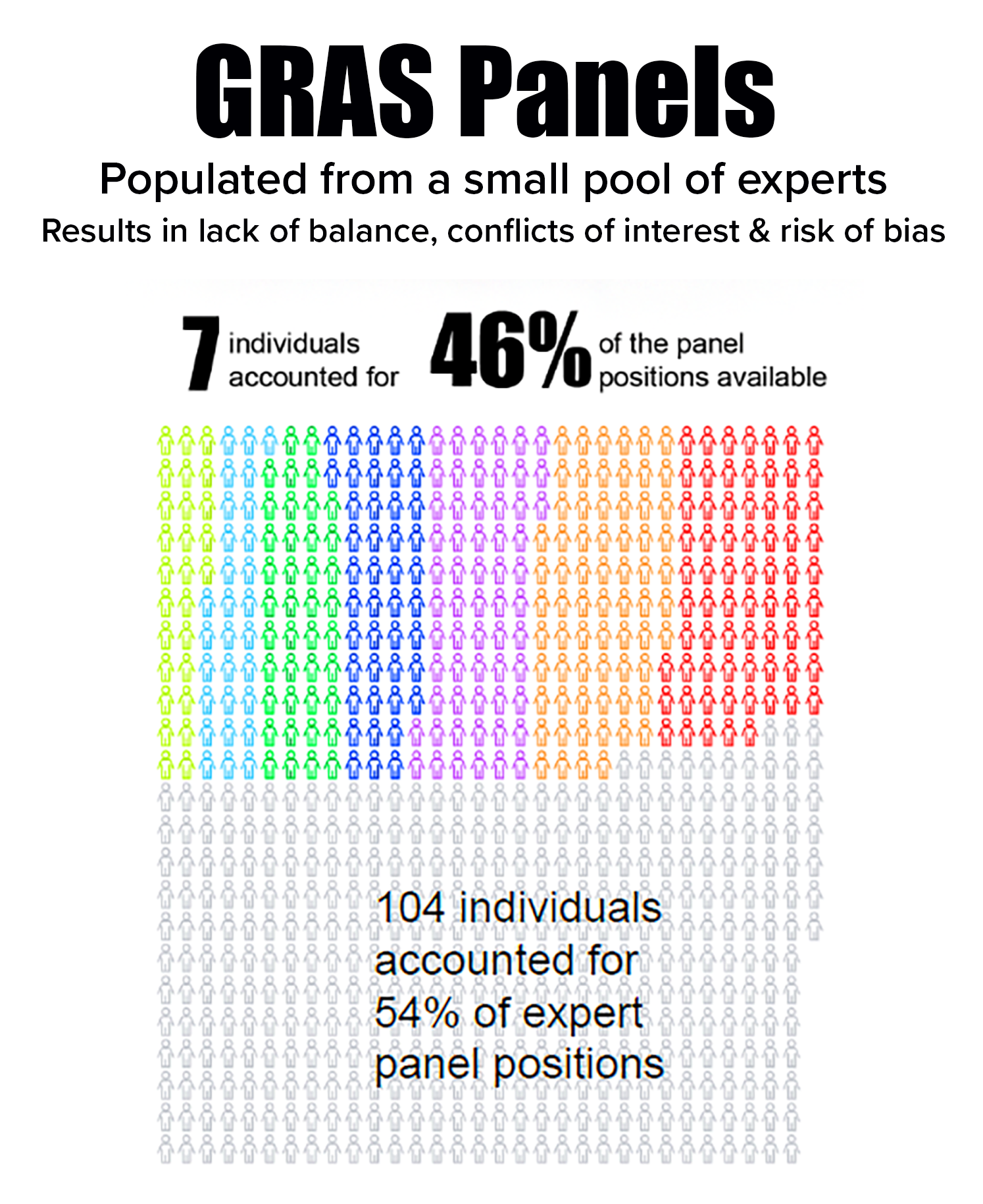
Our study found that no GRAS notices followed the draft guidance. Specifically, we also found there were high risks of bias and conflicts of interest because the companies:
- Had a role—either directly or through a hired third party—in
selecting panelists that likely resulted in bias and conflicts of interest. - Depended on a small pool of experts in which seven individuals occupied 46% of panel positions. The seven often served together, further enhancing risk of bias.
- Relied on panels that did not realistically reflect the diverse scientific community that evaluates chemical risks to public health—which is needed to comply with the law’s requirement that there be a “general recognition” within that community that a substance is GRAS.











