Richard Denison, Ph.D., is a Lead Senior Scientist.
[I delivered these comments at the July 28, 2021 webinar titled “Toxic Chemicals, Whistleblowers, and the Need for Reform at EPA”
cosponso red by Public Employees for Environmental Responsibility (PEER), NY PIRG, and EDF. [A recording of the webinar is available here.] The webinar followed on whistleblower disclosures in a complaint filed by PEER and the first in what will be a series of articles by Sharon Lerner in The Intercept detailing the allegations.]
red by Public Employees for Environmental Responsibility (PEER), NY PIRG, and EDF. [A recording of the webinar is available here.] The webinar followed on whistleblower disclosures in a complaint filed by PEER and the first in what will be a series of articles by Sharon Lerner in The Intercept detailing the allegations.]
I have long described the EPA new chemicals program as a “black box.” For decades, it has operated almost entirely out of public view, in multiple respects:
- Excessive confidentiality claims and withholding of information from the public have been standard operating procedures.
- A purely bilateral mode of operating developed, where the only parties in the room are EPA and the chemical industry.
- The inability of the public to access information and meaningfully participate has severely limited public input and scrutiny.
- As a result, a highly insular, almost secretive program culture arose over time, one where EPA has often viewed its only stakeholders to be the companies seeking quick approval of their new chemicals.
- In sum, private interests trumped public interests.
TSCA reform sought to address key problems
The 2016 amendments to TSCA significantly overhauled the new chemicals provisions of the then-40-year-old law, seeking to rebalance those interests to some extent:
- EPA for the first time is required to make a safety finding for each new chemical and explain the scientific basis for its finding.
- Lack of sufficient information in and of itself is grounds for restricting a chemical and/or requiring testing. Before, unless EPA had enough data to show potential risk, it simply dropped the chemical from further review and allowed it onto the market.
- Companies’ ability to simply assert their submissions are confidential has been reined in in several ways.
To be sure, the amendments did not address all of the program’s problems. For example, despite the fact that the vast majority of new chemicals lack basic safety data, requiring companies to provide a minimum set of information – as many other countries do for new chemicals – was a bridge too far in the face of massive industry opposition. The revelations indicate this is still a big problem: Despite TSCA’s mandate that EPA restrict or require testing of chemicals lacking sufficient information, that has not been happening. EPA still excessively relies on estimating a new chemical’s potential risks using models and extrapolations of data from other chemicals – approaches that have serious limitations, introduce large uncertainties, and are themselves a black box.
Enter the Trump EPA – the damage done
Immediately after the 2016 reforms, there were signs that EPA was starting down a better path. But under the last Administration that progress was quickly reversed and the worst features of the pre-reform program came roaring back. Indeed, where the program ended up was worse than before TSCA reform. Clearly, the new revelations vividly show that – and how far we have to go, both in implementing the reforms and in changing the disturbing culture that still pervades the program. What strikes me about the whistleblowers’ allegations is that they all cut in industry’s favor, removing or downplaying risks the scientists had flagged. This argues against these simply being cases of scientific disagreement and points to a systemic problem.
Let me share with you at a macro level a few numbers that show just how bad things have gotten. In 2017, then-EPA Administrator Scott Pruitt, responding to all-out pressure from the chemical industry, announced EPA would make sweeping changes to new chemical reviews. Many of these sharply deviated from TSCA’s requirements and available evidence. These changes hit the ground in mid-2018.
Since Pruitt’s policy changes, EPA greenlighted most new chemicals:
- Nearly ¾ of new chemicals reviewed (>425 chemicals) were granted unfettered market access – with no conditions imposed.
- Testing requirements entirely disappeared, despite most new chemicals still having no health or environmental safety data.
Let’s look a little closer at just how dramatic a shift this was.
Two main outcomes of new chemicals reviews are possible: either EPA issues a “consent order” for the chemical that imposes conditions or testing requirements; or it issues a determination that the chemical is “not likely to present an unreasonable risk,” in which case the company is free to market the chemical without any conditions.
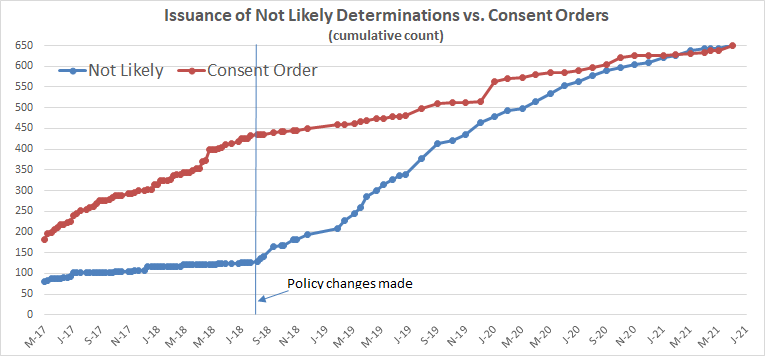
The chart below tracks EPA’s own statistics on the outcomes of its new chemicals reviews from May of 2017 (when EPA first began reporting the numbers) through June of this year.
The summer 2018 inflection point is impossible to miss: The issuance of orders flattened while “not likely” determinations soared – the inverse of what had transpired up to that point in time.
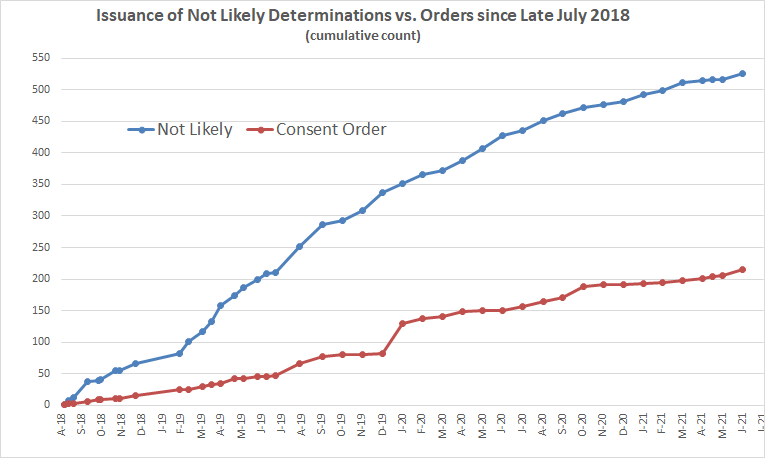
Let’s now zoom in to look at the period after those policy changes took hold.
Nearly three-quarters of all new chemicals reviewed since mid-2018 after Pruitt’s policy changes got “not likely” determinations that cleared them to enter commerce without any conditions.
The second major change Pruitt put in motion was to find a way to dismiss worker risks, no matter how large. This change met a key demand of industry to keep EPA out of workplaces, despite TSCA’s mandate that EPA must ensure protection of workers from chemical risks. For nearly 80% of greenlighted chemicals, EPA identified some risk to workers – but dismissed it by falsely assuming workers would always wear personal protective equipment, or PPE. Of the 80 most recent cases:
- >½ exceeded EPA’s own “acceptable risk” level, and not by only a little bit.
- Dermal risk averaged 15x higher.
- Inhalation risk averaged 4x higher.
Fundamental change is needed
Unfortunately, changes in these trends are not yet apparent, six months into the Biden administration – though in recent months EPA has announced its intent to reverse some of the most damaging of Pruitt’s policy changes.
But EPA’s practice also needs to change. As I said earlier, what the whistleblowers are revealing is what happens when a program operates out of public view.
Here are some things that need to be done to start to turn things around:
- EPA needs to again provide the public with its scientists’ initial recommended decision on each new chemical. EPA had done that for decades but abruptly stopped doing so in January 2018, in a move I characterized as “hiding its tracks.” The public has a right to know if EPA scientists flag concerns based on a company’s new chemical notice; if further information or imposition of conditions addresses that concern, EPA should be clear about what changed. Restoring this simple longstanding practice is vital to increasing the accountability of the review process.
- The public needs to have timely access to all documents related to each new chemical, subject only to redaction of information that is both eligible and meets all TSCA requirements for withholding as confidential. These documents include:
- new chemical notices and all attachments, including any revisions to those documents made during the course of the review;
- all health and safety information on the chemical, which is not eligible to be claimed confidential under TSCA;
- all correspondence between EPA and the notice submitter; and
- the reports and other documents EPA itself develops during the review process to support its decision.
- All versions of new chemical assessments need to be kept and the basis for any changes made to them fully documented and justified.
- A meaningful opportunity for the public to provide input into EPA decisions on new chemicals needs to be provided. This is critical to begin to dismantle the strictly bilateral, behind-closed-doors mode of operation of the program.
- Finally, a thorough investigation is needed of whether program managers used their positions to coerce agency scientists, improperly override their assessments, or sideline or retaliate against them. If so, those managers should not be permitted to retain their positions of authority. Restoring scientific integrity requires holding accountable those who abused it.










3 Comments
How many direct water and sewage “treatment” products were “approved” to be added directly into these systems over these last several decades?
How many direct water and sewage “treatment” products were “approved” to be added directly into these systems over these last several decades without reputable scientists evaluating them?
Please evaluate the “testing procedures” at the NSF.org who has “Nationally Approved” drinking water and waste water “treatment products” to be added directly into these systems. I have found DOI documents that prove these “treatment products” are toxic to these systems, US and our environment. Please feel free to contact me at (262) 613-3263 or at littlplumbergirl@aol.com with any information you may need to prove this and help me get these “treatment products” into the hands of our unbiased, trusted, and reputable scientific communities’ hands who can prove many of these “ingredients” are extremely
toxic to us. Thank you. Sincerely,
Jill Baillargeon