New Chemicals Rule: EPA must require more info from industry
By Maria J. Doa, PhD, Senior Director, Chemicals Policy, and Greg Schweer, Consultant
NOTE: This is the third in a series about EPA’s regulation of new chemicals. See below under Go Deeper for links to other blogs in the series.
What Happened?
EPA recently proposed regulations to govern how it reviews companies’ pre-manufacture notifications for new chemicals before those chemicals can go on the market.
Why It Matters
Industry often waits until late in the review process to submit information—which means that EPA may spend a significant amount of time and effort to revise its risk assessments to incorporate the new information.
EPA has a major opportunity to improve the New Chemicals Program as it crafts these revised regulations. Requiring industry to provide additional “known or reasonably ascertainable information” as required by the law is an important component of this rule. This should reduce the amount of assessment “rework” the agency currently conducts.
Our Take
EDF supports the aspects of EPA’s proposal that clarify, strengthen, and expand on the information required in the initial industry submissions on potential uses, exposures, releases, etc. We also commend EPA for moving to increase efficiency by requiring a more robust submission at the beginning of the review process.
The specific information EPA is proposing to require is information companies already have but generally don’t include in their initial submissions. A company often submits this information only after EPA’s review has identified a risk.
While we support EPA’s proposed requirement that industry submit more complete information, we noted in our comments to EPA [PDF, 721KB] that the proposal could go farther in implementing changes needed to ensure the safety of new chemicals and protect human health and the environment, including those people at greatest potential risk.
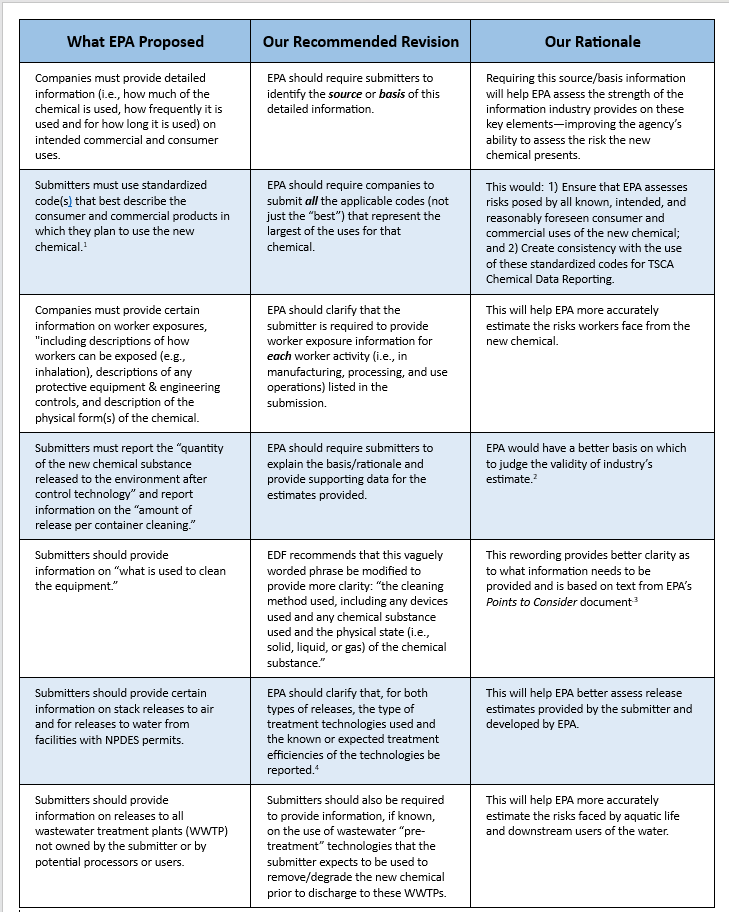
We encourage EPA to strengthen these requirements further by adding additional information requirements to seven of the agency’s proposed changes:
NOTES
1 Organisation for Economic Co-operation and Development, Health and Safety Publications. (2017, May 17). Internationally Harmonised Functional, Product and Article Use Categories. Paris: OECD Environment, Series on Testing & Assessment ENV/JM/MONO(2017)14, No. 262.https://one.oecd.org/document/ENV/JM/MONO(2017)14/en/pdf
2 Environmental Protection Agency, Office of Pollution Prevention and Toxics. (2018, June). Points to Consider When Preparing TSCA New Chemical Notifications. Washington, DC: OMB Control No.: 2070-0012. https://www.epa.gov/sites/default/files/2018-06/documents/points_to_consider_document_2018-06-19_resp_to_omb.pdf
3 This information should be known or reasonably ascertainable and is similar to what EPA has long required for Toxics Release Inventory reporting.
4 Although such information may be available to EPA from the Clean Water Act and Clean Air Act permit number(s) identified by the submitter, it would require extra effort on EPA’s part to find that information and it may not be obvious from the permit.
Go Deeper
Read our previous blogs on EPA’s New Chemicals program.
Follow these links to read the other blogs in the series:
- #1: Time for a New Age for New Chemicals
- #2: EPA: Now’s Your Chance to Get Foxes Out of the Henhouse
- #4: EPA’s New Chemical Regulations: Industry Bias Must Be Fixed
- #5: EPA’s New Chemical Regulations: Backtracking on PBTs
- #6: EPA’s New Chemical Review Process: A Thought Experiment
Download a PDF of this blog post. (PDF, 256KB)