Tom Neltner, J.D., Chemicals Policy Director and Maricel Maffini, Ph.D., Consultant
Update: FDA published the citizen petition upon receipt on 9/23, and is requesting public comment.
More than 60 years ago, Congress enacted legislation requiring the Food and Drug Administration (FDA) and the food industry to evaluate the cumulative effects of substances in the diet that have related health impacts when assessing the safety of chemical additives. In our decade of analyzing FDA and industry actions, we have been increasingly concerned that both have ignored this requirement. To figure it out, we investigated all safety determinations contained in Generally Recognized as Safe (GRAS) notifications voluntarily submitted by food manufacturers to FDA since the program began in 1997. We looked at GRAS notices because they are publicly available and because FDA rules explicitly require that food manufacturers include in the notice an explanation of how they considered the requirement. If there was an omission, it would be more easily noticeable.
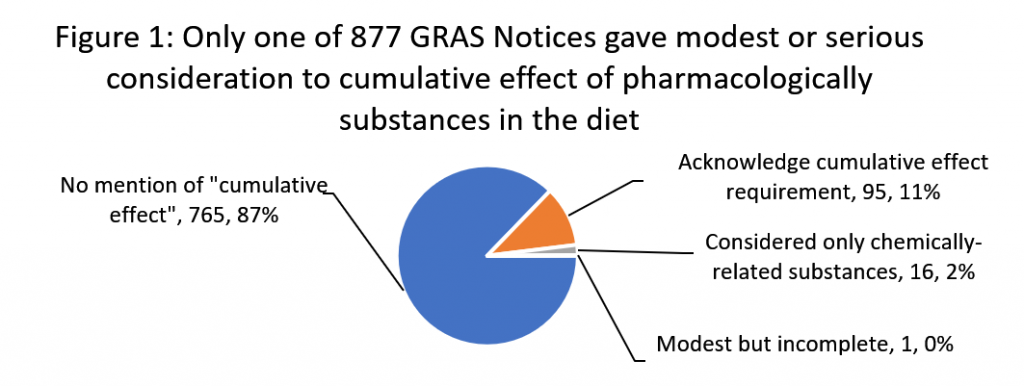
We found that in only one of 877 GRAS notices did a food manufacturer consider the cumulative effect requirement in a meaningful way. And we found no evidence that the agency either recognized this single attempt to follow the law or had objected to the omissions in the 876 other notices. This failure has significant consequences for public health, particularly for communities who already face significant health and socio-economic disparities, and for children, who are uniquely susceptible to dietary exposures to multiple chemicals.
For this reason, EDF joined with other health, environmental, and consumer groups to file a formal petition to demand FDA and food manufacturers start following the law. The petition requests specific changes to rules designed to reinforce the existing requirement and make it easier to verify compliance. Still, given the lack of transparency in agency reviews, success still largely depends on FDA and the food industry taking seriously the mandate and the food safety implications.
The requirement to evaluate cumulative health effects
When Congress enacted the Food Additives Amendment of 1958, it required that FDA consider three issues when evaluating the safety of additives. Among them, the agency was directed to consider “the cumulative effect of such additive in the diet of man or animals, taking into account any chemically or pharmacologically related substance or substances in such diet.”[1]
Six months later, FDA adopted a rule explaining how the agency and food manufacturers must conduct the required review for pharmacologically-related substances. In 1971, it explicitly incorporated the Congressional mandate into its definition of safety. Today, all safety determinations for chemical additives, whether GRAS substances, color additives, food additives, or food contact substances must consider the cumulative health effect of related substances.
EDF’s analysis of GRAS notices
We downloaded 877 generally recognized as safe (GRAS) safety decisions from FDA’s GRAS notice inventory as of March 24, 2020 and analyzed them for terms “cumulative effect” and “pharmacological.” Figure 1 below summarizes our findings.
Only one GRAS notice, GRN 107 for polydextrose, conducted a modest, but incomplete evaluation. It identified 11 pharmacologically-related substances in the diet based on their capacity to have laxative effect. However, after considering the average daily amount at which half of the tested subjects experience laxation of the substances, the notice stopped short of establishing a tolerance for the class as required by FDA rules and only considered the effect from polydextrose alone on the risk of laxation symptoms.
FDA and industry have neglected their responsibility to evaluate cumulative health effects
While not as immediate as adverse drug interactions or food poisoning, the long-term, combined impact of food chemicals on our health can be significant. For example, several food additives and contaminants in common foods – including nitrates, perchlorate, and thiocyanate – all harm the thyroid’s ability to use iodine to make a hormone essential to brain development. Exposure to these related chemicals from various foods should be considered together, as a class, to reduce the risk for pregnant women and young children, as the chemicals all harm fetal and infant brain development in the similar way.
Both industry and the FDA all too often consider one chemical at a time when evaluating how they impact our health – rather than as a class of related substances, as the agency’s regulations state. The collective failure by FDA and the food industry to follow the law may well have contributed to the dramatic increases we have seen in chronic diseases, including obesity, diabetes, and kidney disease in the US in recent decades. Every time we eat highly processed food, we are exposed to chemical additives, and – when the chemicals cause similar toxic effects – that exposure can harm our health. Regulating chemicals with similar toxicity as a class would reduce the overall exposure to those substances within the class, thus reducing health risks.
The petition outlines the necessary steps for FDA to take to ensure that the cumulative effect of a new substance and related substances already in the diet has been adequately evaluated before the new additive is allowed in our food. The petitioners are asking that the agency update its rules, issue guidance to industry to explain what is needed to conduct a complete safety determination which includes accounting for cumulative effects and revise its forms for industry to submit notices and petitions.
The petitioners are: EDF; American Academy of Pediatrics; American Public Health Association; Breast Cancer Prevention Partners; Center for Food Safety; Clean Label Project; Consumer Federation of America; Consumer Reports; Endocrine Society; Environmental Health Strategy Center; Environmental Working Group; and Healthy Babies, Bright Futures.
It’s time FDA followed the law and protect American families from the cumulative health effects of chemicals in the foods we eat every day.
See the press release for the petition here.
[1] 21 U.S.C. § 348(c)(5)(B)