Richard Denison, Ph.D., is a Lead Senior Scientist.
I blogged last week about the Environmental Protection Agency’s (EPA) illegal and hypocritical decision to deny the public access to health and safety studies conducted on the first chemical to undergo a risk evaluation under the reformed Toxic Substances Control Act (TSCA). In its draft risk evaluation, now out for public comment, EPA relied on these secret studies to assert that the chemical, commonly known as Pigment Violet 29, or PV29, is safe, so EPA’s denial of public access matters a great deal.
EPA asserts that these studies are entitled to protection as confidential business information (CBI) under TSCA, when in fact TSCA explicitly does not extend CBI protection to such studies. The only health and environmental information on this chemical that is public are brief summaries of those studies that were prepared by the companies that make the chemical, and were submitted to the European Chemicals Agency (ECHA) when the chemical was registered under the European Union’s REACH Regulation. (EPA erroneously states that the studies were “summarized by ECHA.” This is simply not the case: Registrants, not ECHA, develop the summaries that are then made available in the registration “dossiers” for REACH chemicals.)
As we review EPA’s draft risk evaluation for PV29, we are finding that EPA’s assertions cannot be trusted even about what these summaries state are the findings of the underlying studies. I’ll discuss one such case in this post.
In the Executive Summary of its draft risk evaluation, EPA asserts: “The human health testing reported that no adverse effects were observed for all routes of exposure (oral, dermal, inhalation).” And after listing the studies on which it relies, EPA asserts on page 25: “These full study reports concluded that no adverse effects were observed for all routes of exposure (oral, dermal, inhalation). … As a result, the EPA concludes that C.I. Pigment Violet 29 presents a low hazard to human health.” As I noted in my last post, EPA has reached these conclusions despite very limited data on PV29, and no data at all relating to chronic effects.
Two of those studies are identified by EPA as follows: “Non-Guideline Acute Toxicity: Acute Inhalation Toxicity with Rats (two studies).” These studies, conducted by BASF four decades ago (in 1976 and 1978), are the sole basis EPA cites for its assertion that “no adverse effects were observed for” the inhalation route of exposure.
So imagine our surprise when we examined the summaries of these two studies and found that a wholly different conclusion was drawn by BASF, the company that conducted the studies and submitted them in 2013 to ECHA under REACH:
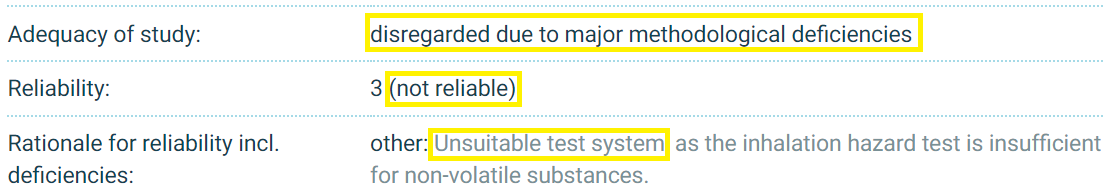
To repeat: BASF itself labeled these two studies “not reliable” due to use of an “unsuitable test system” and said the studies should be “disregarded due to major methodological deficiencies.”
(See for yourself: The first summary is available both here in EPA’s docket and here in the REACH dossier. The second summary is available both here in EPA’s docket and here in the REACH dossier. Both are also listed in Appendix D of EPA’s draft risk evaluation.)
Yet, as noted in Appendix D, EPA ranked these two studies as of “medium” quality, and proceeded to rely exclusively on them to conclude PV29 poses absolutely no hazard or risk from inhalation. How did EPA achieve this miraculous resurrection of these “not reliable” studies? By applying the dark magic of its TSCA Systematic Review approach. Readers may recall that this approach is being used in all of the first 10 risk evaluations being conducted under TSCA – despite the fact that it deviates in major respects from all other authoritative systematic review protocols and has never been subject to any independent scientific peer review. EDF earlier noted these deficiencies, among many other concerns, in our extensive comments on EPA’s TSCA Systematic Review document.
It took quite a bit of digging to ferret out what I report in this blog post. I can only imagine what other mischief lies beneath the surface of EPA’s first draft risk evaluation, as well as the other nine that are soon to come. All of this . . . despite a new law that demands EPA use the best available science when evaluating chemical risks.
Is it any wonder that EDF and other groups have filed a Freedom of Information Act (FOIA) request for all of the full studies on PV29?









