Richard Denison, Ph.D., is a Lead Senior Scientist. Lindsay McCormick is a Project Manager.
Today, EPA issued its long-awaited rule to gather risk-relevant information on nanoscale materials. The new rule will finally allow EPA to obtain basic data on use, exposure, and hazards from those that manufacture or process these materials, which has long been recognized by experts as essential to understand and manage their potential risks.
Nanomaterials – a diverse category of materials defined mainly by their small size – often exhibit unique properties that can allow for novel applications but also have the potential to negatively impact our health and the environment. Some nanomaterials: more easily penetrate biological barriers than do their bulk counterparts; exhibit toxic effects on the nervous, cardiovascular, pulmonary, and reproductive systems; or have antibacterial properties that may negatively impact ecosystems or lead to resistance.
Numerous expert bodies have identified the need for the kinds of information on nanomaterials EPA will now be able to collect, including the National Academy of Sciences, the National Nanotechnology Initiative, and EPA’s Office of Research and Development. The Organization for Economic Cooperation and Development (OECD) published a report last year noting that the number of products containing nanomaterials increased fivefold in the global market between 2006 and 2011, and are being used in hundreds of new products ranging from cosmetics and personal care products to clothing and textiles, solar cells, plastics for the automotive and aircraft industries, and food packaging.
EPA’s new rule institutes a one-time reporting requirement for existing nanomaterials, as well as a standing one-time reporting requirement for new nanomaterials before they are manufactured. Companies that manufacture, import or process existing nanomaterials, or intend to start doing so for a new nanomaterial, are required to submit the following categories of “reasonably ascertainable” information to EPA: chemical identity, production volume, methods of manufacture and processing, exposure and release information, and available environmental and health impacts data. By collecting such data, EPA will finally be able to draw a clearer picture of the nanomaterials in and entering commercial use, and better determine whether action to mitigate risk is needed, on a case-by-case basis.
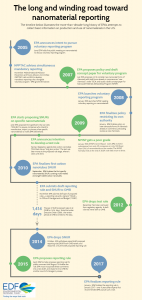
This basic rule has been a very long time coming. As illustrated in the graphic above, EPA has been attempting – for over a decade – to issue such a rule to gather even this most basic information on nanomaterials in the U.S. market. Over the years, we have blogged extensively on EPA’s slow progress, due to opposition from both industry and other parts of the Federal government at every step.
There are a number of notable aspects of what is included – and not included – in the final rule:
- It applies both to nanoscale materials already in commerce and to new nano forms of existing chemicals that companies intend to make or process in the future. (Wholly new nanoscale chemicals would be required to be reviewed under TSCA’s new chemicals provisions.)
- In promulgating the rule, EPA affirmed its broad authority to collect existing information under section 8(a) of TSCA, rejecting industry arguments that such authority was highly constrained.
- EPA removed reporting exemptions it had proposed for nanoclays and zinc oxide, and rejected industry calls to include numerous additional exemptions.
The rule is not perfect and omits reporting EDF and others urged be included. For example:
- Aggregates of nanoscale particles must fall within the 1-100 nanometer (nm) range to be reportable. We argued that aggregates comprised of nanoparticles between 1-100 nm be reported even if the aggregate is larger, given that such aggregates can often disaggregate in the environment or during use.
- Companies that submitted a pre-manufacture notice (PMN) for a nanoscale material at any time since 2005 do not have to report for that material. Our concern is that this will miss new information on that material that has been developed since the PMN was reviewed.
- Chemical substances “formed at the nanoscale as part of a film on a surface” are exempted from reporting. We argued that such films can break down or erode over time especially if exposed to the elements, potentially releasing the nanoscale materials.
Still, with this rule finally finalized, EPA can at last begin to get basic risk-relevant information needed to make sound decisions about which materials and uses present concerns and which do not.
For more detail on the history of this rule, see these earlier blog posts:
- Nano Confessions: EPA all but concedes mandatory reporting and testing are needed (January, 2009)
- Waiting for Godot: 405 days and counting at OMB on EPA’s modest proposal to identify chemicals of concern under TSCA (June, 2011)
- A hint of movement in the Super Slo-Mo that is nanoregulation at EPA under TSCA (October, 2014)
- Why is the nanotech industry so intent on keeping EPA from doing its job? (August, 2015)